High efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Muheza and Kigoma Districts, Tanzania
- PMID: 29996849
- PMCID: PMC6042436
- DOI: 10.1186/s12936-018-2409-z
High efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Muheza and Kigoma Districts, Tanzania
Abstract
Background: Artemether-lumefantrine (AL) is the recommended first-line artemisinin-based combination therapy (ACT) for the treatment of uncomplicated falciparum malaria in most of the malaria-endemic countries, including Tanzania. Recently, dihydroartemisinin-piperaquine (DP) has been recommended as the alternative anti-malarial to ensure effective case management in Tanzania. This study assessed the parasite clearance rate and efficacy of AL and DP among patients aged 6 months to 10 years with uncomplicated falciparum malaria in two sites with different malaria transmission intensity.
Methods: This was an open-label, randomized trial that was conducted at two sites of Muheza Designated District Hospital and Ujiji Health Centre in Tanga and Kigoma regions, respectively. Patients meeting inclusion criteria were enrolled, treated with either AL or DP and followed up for 28 (extended to 42) and 42 (63) days for AL and DP, respectively. Parasite clearance time was monitored in the first 72 h post treatment and the clearance rate constant and half-life were calculated using an established parasite clearance estimator. The primary outcome was parasitological cure on days 28 and 42 for AL and DP, respectively, while secondary outcome was extended parasitological cure on days 42 and 63 for AL and DP, respectively.
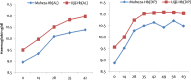
Results: Of the 509 children enrolled (192 at Muheza and 317 at Ujiji), there was no early treatment failure and PCR uncorrected cure rates on day 28 in the AL group were 77.2 and 71.2% at Muheza and Ujiji, respectively. In the DP arm, the PCR uncorrected cure rate on day 42 was 73.6% at Muheza and 72.5% at Ujiji. With extended follow-up (to day 42 for AL and 63 for DP) cure rates were lower at Ujiji compared to Muheza (AL: 60.2 and 46.1%, p = 0.063; DP: 57.6 and 40.3% in Muheza and Ujiji, respectively, p = 0.021). The PCR corrected cure rate ranged from 94.6 to 100% for all the treatment groups at both sites. Parasite clearance rate constant was similar in the two groups and at both sites (< 0.28/h); the slope half-life was < 3.0 h and all but only one patient cleared parasites by 72 h.
Conclusion: These findings confirm high efficacy of the first- and the newly recommended alternative ACT for treatments for uncomplicated falciparum malaria in Tanzania. The high parasite clearance rate suggests absence of suspected artemisinin resistance, defined as delayed parasite clearance. Trial registration This trial is registered at ClinicalTrials.gov under registration number NCT02590627.
Keywords: Artemether–lumefantrine; Dihydroartemisinin–piperaquine; Efficacy; Parasite clearance; Plasmodium falciparum.
Figures
Similar articles
-
High cure rates and tolerability of artesunate-amodiaquine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Kibaha and Kigoma, Tanzania.Malar J. 2019 Mar 25;18(1):99. doi: 10.1186/s12936-019-2740-z. Malar J. 2019. PMID: 30909922 Free PMC article.
-
Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania.Malar J. 2018 Oct 17;17(1):369. doi: 10.1186/s12936-018-2524-x. Malar J. 2018. PMID: 30333022 Free PMC article.
-
Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria and prevalence of molecular markers associated with artemisinin and partner drug resistance in Uganda.Malar J. 2021 Dec 24;20(1):484. doi: 10.1186/s12936-021-04021-5. Malar J. 2021. PMID: 34952573 Free PMC article.
-
Paediatric formulations of artemisinin-based combination therapies for treating uncomplicated malaria in children.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD009568. doi: 10.1002/14651858.CD009568.pub2. Cochrane Database Syst Rev. 2020. PMID: 33289099 Free PMC article.
-
Efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Aug 12;20(1):340. doi: 10.1186/s12936-021-03873-1. Malar J. 2021. PMID: 34384431 Free PMC article.
Cited by
-
Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis.PLoS One. 2022 Mar 10;17(3):e0264339. doi: 10.1371/journal.pone.0264339. eCollection 2022. PLoS One. 2022. PMID: 35271592 Free PMC article.
-
High therapeutic efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Somalia.Malar J. 2019 Jul 11;18(1):231. doi: 10.1186/s12936-019-2864-1. Malar J. 2019. PMID: 31296223 Free PMC article. Clinical Trial.
-
Genetic polymorphism and evidence of signatures of selection in the Plasmodium falciparum circumsporozoite protein gene in Tanzanian regions with different malaria endemicity.medRxiv [Preprint]. 2024 Jan 23:2024.01.23.24301587. doi: 10.1101/2024.01.23.24301587. medRxiv. 2024. Update in: Malar J. 2024 May 8;23(1):139. doi: 10.1186/s12936-024-04974-3. PMID: 38343796 Free PMC article. Updated. Preprint.
-
Efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in mainland Tanzania, 2019.Malar J. 2024 Apr 9;23(1):101. doi: 10.1186/s12936-024-04931-0. Malar J. 2024. PMID: 38594679 Free PMC article.
-
Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations for uncomplicated malaria in 10 sentinel sites across Ghana: 2015-2017.Malar J. 2019 Jun 24;18(1):206. doi: 10.1186/s12936-019-2848-1. Malar J. 2019. PMID: 31234874 Free PMC article.
References
-
- WHO . World malaria report. Geneva: World Health Organization; 2017.
-
- WHO . Global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva: World Health Organization; 2010.
-
- Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: dihydroartemisinin–piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical