Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting
- PMID: 29996864
- PMCID: PMC6042421
- DOI: 10.1186/s12969-018-0255-8
Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting
Abstract
Background: Conventional pharmacological therapies for the treatment of juvenile idiopathic arthritis (JIA) consist of non-biological, disease-modifying antirheumatic drugs, among which methotrexate (MTX) is the most commonly prescribed. However, there is a lack of consensus-based clinical and therapeutic recommendations for the use of MTX in the management of patients with JIA. Therefore, the Methotrexate Advice and RecommendAtions on Juvenile Idiopathic Arthritis (MARAJIA) Expert Meeting was convened to develop evidence-based recommendations for the use of MTX in the treatment of JIA.
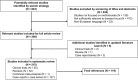
Methods: The preliminary executive committee identified a total of 9 key clinical issues according to the population, intervention, comparator, outcome (PICO) approach, and performed an evidence-based, systematic, literature review. During the subsequent Expert Meeting, the relevant evidence was assessed and graded, and 10 recommendations were made.
Results: Recommendations relating to the efficacy, optimal dosing and route of administration and duration of treatment with MTX in JIA, and to the issue of folic acid supplementation to prevent MTX side effects, use of MTX in the treatment of chronic JIA-associated uveitis, combination treatment with biologic agents, and the use of vaccinations in patients with JIA were developed. The selected topics were considered to represent clinically important issues facing clinicians caring for patients with JIA. Evidence was insufficient to formulate recommendations for the use of biomarkers predictive of treatment response.
Conclusions: These consensus recommendations provide balanced and evidence-based recommendations designed to have broad value for physicians and healthcare clinicians involved in the clinical management of patients with JIA.
Keywords: Consensus; Juvenile idiopathic arthritis; Methotrexate.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable. No patient data was included in this work.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. - PubMed
-
- Calvo I, Anton J, Lopez Robledillo JC, de Inocencio J, Gamir ML, Merino R, et al. Recommendations for the use of methotrexate in patients with juvenile idiopathic arthritis. An Pediatr (Barc) 2016;84(177):e1–e8. - PubMed
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from https://handbook.cochrane.org..
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical