Planning a future randomized clinical trial based on a network of relevant past trials
- PMID: 29996869
- PMCID: PMC6042258
- DOI: 10.1186/s13063-018-2740-2
Planning a future randomized clinical trial based on a network of relevant past trials
Abstract
Background: The important role of network meta-analysis of randomized clinical trials in health technology assessment and guideline development is increasingly recognized. This approach has the potential to obtain conclusive results earlier than with new standalone trials or conventional, pairwise meta-analyses.
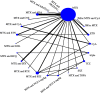
Methods: Network meta-analyses can also be used to plan future trials. We introduce a four-step framework that aims to identify the optimal design for a new trial that will update the existing evidence while minimizing the required sample size. The new trial designed within this framework does not need to include all competing interventions and comparisons of interest and can contribute direct and indirect evidence to the updated network meta-analysis. We present the method by virtually planning a new trial to compare biologics in rheumatoid arthritis and a new trial to compare two drugs for relapsing-remitting multiple sclerosis.
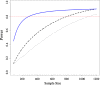
Results: A trial design based on updating the evidence from a network meta-analysis of relevant previous trials may require a considerably smaller sample size to reach the same conclusion compared with a trial designed and analyzed in isolation. Challenges of the approach include the complexity of the methodology and the need for a coherent network meta-analysis of previous trials with little heterogeneity.
Conclusions: When used judiciously, conditional trial design could significantly reduce the required resources for a new study and prevent experimentation with an unnecessarily large number of participants.
Keywords: Conditional power; Evidence synthesis; Historical data; Rheumatoid arthritis; Sample size.
Conflict of interest statement
Ethics approval and consent to participate
Not required.
Consent for publication
Not required.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Synthesizing existing evidence to design future trials: survey of methodologists from European institutions.Trials. 2019 Jun 7;20(1):334. doi: 10.1186/s13063-019-3449-6. Trials. 2019. PMID: 31174597 Free PMC article.
-
Conditional power of antidepressant network meta-analysis.BMC Psychiatry. 2021 Mar 5;21(1):129. doi: 10.1186/s12888-021-03094-5. BMC Psychiatry. 2021. PMID: 33673822 Free PMC article.
-
Empirical assessment suggests that existing evidence could be used more fully in designing randomized controlled trials.J Clin Epidemiol. 2010 Sep;63(9):983-91. doi: 10.1016/j.jclinepi.2010.01.022. Epub 2010 Jun 22. J Clin Epidemiol. 2010. PMID: 20573483 Review.
-
Evaluation of networks of randomized trials.Stat Methods Med Res. 2008 Jun;17(3):279-301. doi: 10.1177/0962280207080643. Epub 2007 Oct 9. Stat Methods Med Res. 2008. PMID: 17925316 Review.
Cited by
-
Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis.JAMA Oncol. 2019 Apr 1;5(4):480-489. doi: 10.1001/jamaoncol.2018.6720. JAMA Oncol. 2019. PMID: 30763436 Free PMC article.
-
Comparative effectiveness and safety of analgesic medicines for adults with acute non-specific low back pain: systematic review and network meta-analysis.BMJ. 2023 Mar 22;380:e072962. doi: 10.1136/bmj-2022-072962. BMJ. 2023. PMID: 36948512 Free PMC article.
-
Redundant trials can be prevented, if the EU clinical trial regulation is applied duly.BMC Med Ethics. 2020 Oct 28;21(1):107. doi: 10.1186/s12910-020-00536-9. BMC Med Ethics. 2020. PMID: 33115456 Free PMC article.
-
Low awareness of the transitivity assumption in complex networks of interventions: a systematic survey from 721 network meta-analyses.BMC Med. 2024 Mar 13;22(1):112. doi: 10.1186/s12916-024-03322-1. BMC Med. 2024. PMID: 38475826 Free PMC article.
-
Network meta-analysis reaches nutrition research.Eur J Nutr. 2019 Feb;58(1):1-3. doi: 10.1007/s00394-018-1849-0. Eur J Nutr. 2019. PMID: 30382331 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

