Splenic vein resection together with the pancreatic parenchyma versus separated resection after isolation of the parenchyma during distal pancreatectomy (COSMOS-DP trial): study protocol for a randomised controlled trial
- PMID: 29996884
- PMCID: PMC6042420
- DOI: 10.1186/s13063-018-2756-7
Splenic vein resection together with the pancreatic parenchyma versus separated resection after isolation of the parenchyma during distal pancreatectomy (COSMOS-DP trial): study protocol for a randomised controlled trial
Abstract
Background: In distal pancreatectomy (DP), it is customary to ligate and divide the splenic vein after isolating it from the pancreatic parenchyma. This is considered essential to prevent disruption of the stump of the splenic vein and consequent intra-abdominal haemorrhage in the event of pancreatic fistula (PF). However, this procedure can be technically demanding, especially when the vein is firmly embedded in the pancreatic parenchyma. The objective of the COSMOS-DP trial is to confirm the non-inferiority of resection of the splenic vein embedded in the pancreatic parenchyma compared with the conventional technique of isolating the splenic vein before resection during DP using a mechanical stapler.
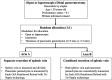
Methods: Patients with diseases of the pancreatic body and tail whose pancreatic parenchyma and splenic vein can be divided concurrently during open or laparoscopic DP are considered eligible for inclusion. This study is designed as a multicentre prospective randomised phase III trial. Eligible patients will be centrally randomised to either Arm A (resection of the splenic vein after isolation from the pancreatic parenchyma) or Arm B (co-resection of the vein together with the pancreas). This study aims to establish the non-inferiority of the safety of Arm B compared with that of Arm A; the primary endpoint is the incidence of PF (ISGPF grade B/C).
Discussion: The COSMOS-DP trial will establish the safety of this procedure, such that it can be recommended with more confidence. The use of this procedure will likely result in significant reductions in operative time and blood loss during DP.
Trial registration: ClinicalTrials.gov, NCT02871804 . Registered on 27 July 2016.
Keywords: Distal pancreatectomy; Intra-abdominal haemorrhage; Mechanical stapler; Pancreatic fistula; Splenic vein.
Conflict of interest statement
Ethics approval and consent to participate
This study will be conducted according to the Declaration of Helsinki (Fortaleza, Brazil, October 2013) and ethical guidelines for medical and health research involving human subjects in Japan. Written informed consent approved by the Institutional Review Board will be obtained from all of the enrolled patients. Ethical approval for this study was obtained by the Institutional Review Board of each institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Safety of Combined Division vs Separate Division of the Splenic Vein in Patients Undergoing Distal Pancreatectomy: A Noninferiority Randomized Clinical Trial.JAMA Surg. 2021 May 1;156(5):418-428. doi: 10.1001/jamasurg.2021.0108. JAMA Surg. 2021. PMID: 33656542 Free PMC article. Clinical Trial.
-
Randomized Controlled Trial of Pancreaticojejunostomy versus Stapler Closure of the Pancreatic Stump During Distal Pancreatectomy to Reduce Pancreatic Fistula.Ann Surg. 2016 Jul;264(1):180-7. doi: 10.1097/SLA.0000000000001395. Ann Surg. 2016. PMID: 26473652 Free PMC article. Clinical Trial.
-
The efficacy of wrapping with polyglycolic acid mesh and fibrin glue in preventing clinically relevant pancreatic fistula after minimally invasive distal pancreatectomy (WRAP Study): study protocol for a multicenter randomized controlled trial in Japan.BMC Surg. 2024 Oct 16;24(1):314. doi: 10.1186/s12893-024-02610-0. BMC Surg. 2024. PMID: 39415231 Free PMC article.
-
Geographical variation and trends in outcomes of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessel preservation: A meta-analysis.Int J Surg. 2017 Sep;45:47-55. doi: 10.1016/j.ijsu.2017.07.078. Epub 2017 Jul 21. Int J Surg. 2017. PMID: 28735894 Review.
-
[Manual suture or stapler closure: management of pancreatic stump during distal pancreatectomy].Zhonghua Wai Ke Za Zhi. 2020 Jul 1;58(7):494-498. doi: 10.3760/cma.j.cn112139-20200410-00289. Zhonghua Wai Ke Za Zhi. 2020. PMID: 32610417 Review. Chinese.
Cited by
-
Considerations in laparoscopic resection of giant pancreatic cystic neoplasms.J Minim Access Surg. 2022 Oct-Dec;18(4):519-525. doi: 10.4103/jmas.jmas_164_21. J Minim Access Surg. 2022. PMID: 35046179 Free PMC article.
-
Pancreatic Fat and Body Composition Measurements by Computed Tomography are Associated with Pancreatic Fistula After Pancreatectomy.Ann Surg Oncol. 2021 Jan;28(1):530-538. doi: 10.1245/s10434-020-08581-9. Epub 2020 May 20. Ann Surg Oncol. 2021. PMID: 32436185
-
The efficacy of polyglycolic acid felt reinforcement in preventing postoperative pancreatic fistula after pancreaticojejunostomy in patients with main pancreatic duct less than 3 mm in diameter and soft pancreas undergoing pancreatoduodenectomy (PLANET-PJ trial): study protocol for a multicentre randomized phase III trial in Japan and Korea.Trials. 2019 Aug 9;20(1):490. doi: 10.1186/s13063-019-3595-x. Trials. 2019. PMID: 31399139 Free PMC article.
References
-
- Frozanpor F, Lundell L, Segersvard R, Arnelo U. The effect of prophylactic transpapillary pancreatic stent insertion on clinically significant leak rate following distal pancreatectomy: results of a prospective controlled clinical trial. Ann Surg. 2012;255:1032–1036. doi: 10.1097/SLA.0b013e318251610f. - DOI - PubMed
-
- Carter TI, Fong ZV, Hyslop T, Lavu H, Tan WP, Hardacre J, et al. A dual-institution randomized controlled trial of remnant closure after distal pancreatectomy: does the addition of a falciform patch and fibrin glue improve outcomes? J Gastrointest Surg. 2013;17:102–109. doi: 10.1007/s11605-012-1963-x. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical