An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial
- PMID: 29996895
- PMCID: PMC6042238
- DOI: 10.1186/s13063-018-2683-7
An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial
Abstract
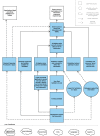
Background: Whilst pathways relating to the early stages of stroke care have become well established, strategies for longer-term care are less developed and longer-term outcomes remain poor for many stroke survivors. New Start, a complex intervention that includes needs identification, exploration of social networks and components of problem-solving and self-management, was designed to improve stroke survivors' quality of life by addressing unmet needs and increasing participation. It is delivered approximately 6 months post-stroke by trained staff (facilitators). We are currently undertaking a cluster randomised feasibility trial of the New Start intervention with an embedded process evaluation, which is an important component of the design and testing of complex interventions as it provides an understanding of how interventions are delivered and function in different settings.
Methods/design: This mixed methods process evaluation will explore the degree to which New Start is implemented as intended, the impact of context on intervention delivery and the acceptability of the intervention for stroke survivors, their families and practitioners. It will include non-participant observation of facilitator training and intervention delivery. Interviews with stroke survivors, facilitators and other relevant staff (including administrators and managerial staff) will be undertaken. Qualitative data from interview transcripts, facilitator reflections and observational field notes will be analysed thematically alongside numerical data documenting intervention delivery collected as part of the trial.
Discussion: This process evaluation will identify factors that aid and impede implementation of the New Start intervention and improve understanding of this novel approach to longer-term stroke care.
Trial registration: ISRCTN Registry, ISRCTN38920246 . Registered on 22 June 2016.
Keywords: Complex intervention; Interviews; Logic model; New Start; Observations; Outcomes chain; Process evaluation; Self-management; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been obtained through the Yorkshire and the Humber – Leeds West Research Ethics Committee (REC) (16/YH/0390).
Ethical approval includes agreed processes for obtaining consent (or consultee declaration) from (or with regard to) all staff, stroke survivors and carers with and without capacity, in line with the provisions of the Mental Capacity Act.
Any protocol amendments will be approved by the REC prior to informing researchers and participating sites of any related process changes. Amendments impacting participants’ involvement will be communicated to them in an appropriate manner, as agreed by the REC. At the time of writing, the current approved protocol is version 2, dated 20 March 2017.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
An intervention to support stroke survivors and their carers in the longer term: results of a cluster randomised controlled feasibility trial (LoTS2Care).Pilot Feasibility Stud. 2023 Mar 15;9(1):40. doi: 10.1186/s40814-023-01258-6. Pilot Feasibility Stud. 2023. PMID: 36922866 Free PMC article.
-
An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial.Trials. 2018 Jun 11;19(1):317. doi: 10.1186/s13063-018-2669-5. Trials. 2018. PMID: 29891011 Free PMC article.
-
Longer-term health and social care strategies for stroke survivors and their carers: the LoTS2Care research programme including cluster feasibility RCT.Southampton (UK): NIHR Journals Library; 2021 Mar. Southampton (UK): NIHR Journals Library; 2021 Mar. PMID: 33819000 Free Books & Documents. Review.
-
Organising Support for Carers of Stroke Survivors (OSCARSS): study protocol for a cluster randomised controlled trial, including health economic analysis.Trials. 2019 Jan 7;20(1):19. doi: 10.1186/s13063-018-3104-7. Trials. 2019. PMID: 30616692 Free PMC article.
-
Strategies to enhance routine physical activity in care home residents: the REACH research programme including a cluster feasibility RCT.Southampton (UK): NIHR Journals Library; 2021 Aug. Southampton (UK): NIHR Journals Library; 2021 Aug. PMID: 34432399 Free Books & Documents. Review.
Cited by
-
Patients' experience of and participation in a stroke self-management programme, My Life After Stroke (MLAS): a multimethod study.BMJ Open. 2022 Nov 15;12(11):e062700. doi: 10.1136/bmjopen-2022-062700. BMJ Open. 2022. PMID: 36379661 Free PMC article. Clinical Trial.
-
An intervention to support stroke survivors and their carers in the longer term: results of a cluster randomised controlled feasibility trial (LoTS2Care).Pilot Feasibility Stud. 2023 Mar 15;9(1):40. doi: 10.1186/s40814-023-01258-6. Pilot Feasibility Stud. 2023. PMID: 36922866 Free PMC article.
References
-
- Intercollegiate Stroke Working Party . National Clinical Guidelines for Stroke. 4. London: Royal College of Physicians; 2012.
-
- Forster A, Hartley S, Barnard L, Ozer S, Hardicre N, Crocker T, Fletcher M, Moreau L, Atkinson R, Hulme C, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomized controlled feasibility trial. Trials. 2017;19(1):317. 10.1186/s13063-018-2669-5. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical