Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation
- PMID: 29996901
- PMCID: PMC6042447
- DOI: 10.1186/s13287-018-0930-1
Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation
Erratum in
-
Correction to: Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation.Stem Cell Res Ther. 2018 Aug 28;9(1):229. doi: 10.1186/s13287-018-0975-1. Stem Cell Res Ther. 2018. PMID: 30153854 Free PMC article.
Abstract
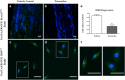
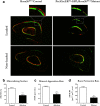
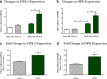
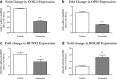
Background: The fully developed adult skeleton adapts to mechanical forces by generating more bone, usually at the periosteal surface. Progenitor cells in the periosteum are believed to differentiate into bone-forming osteoblasts that contribute to load-induced adult bone formation, but in vivo evidence does not yet exist. Furthermore, the mechanism by which periosteal progenitors might sense physical loading and trigger differentiation is unknown. We propose that periosteal osteochondroprogenitors (OCPs) directly sense mechanical load and differentiate into bone-forming osteoblasts via their primary cilia, mechanosensory organelles known to be involved in osteogenic differentiation.
Methods: We generated a diphtheria toxin ablation mouse model and performed ulnar loading and dynamic histomorphometry to quantify the contribution of periosteal OCPs in adult bone formation in vivo. We also generated a primary cilium knockout model and isolated periosteal cells to study the role of the cilium in periosteal OCP mechanosensing in vitro. Experimental groups were compared using one-way analysis of variance or student's t test, and sample size was determined to achieve a minimum power of 80%.
Results: Mice without periosteal OCPs had severely attenuated mechanically induced bone formation and lacked the mineralization necessary for daily skeletal maintenance. Our in vitro results demonstrate that OCPs in the periosteum uniquely sense fluid shear and exhibit changes in osteogenic markers consistent with osteoblast differentiation; however, this response is essentially lost when the primary cilium is absent.
Conclusions: Combined, our data show that periosteal progenitors are a mechanosensitive cell source that significantly contribute to adult skeletal maintenance. More importantly, an OCP population persists in the adult skeleton and these cells, as well as their cilia, are promising targets for bone regeneration strategies.
Keywords: Bone regeneration; Mechanotransduction; Osteoblasts; Osteochondroprogenitors; Periosteal progenitors; Primary cilium; Prx1.
Conflict of interest statement
Ethics approval
All animal studies were approved by the Institute of Comparative Medicine at Columbia University and experiments were conducted in accordance with national IACUC standards. Animals were housed and cared for in an AAALAC certified barrier facility.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Prx1-Expressing Progenitor Primary Cilia Mediate Bone Formation in response to Mechanical Loading in Mice.Stem Cells Int. 2019 Nov 11;2019:3094154. doi: 10.1155/2019/3094154. eCollection 2019. Stem Cells Int. 2019. PMID: 31814831 Free PMC article.
-
αSMA Osteoprogenitor Cells Contribute to the Increase in Osteoblast Numbers in Response to Mechanical Loading.Calcif Tissue Int. 2020 Feb;106(2):208-217. doi: 10.1007/s00223-019-00624-y. Epub 2019 Oct 31. Calcif Tissue Int. 2020. PMID: 31673746 Free PMC article.
-
Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone.Bone. 2017 Oct;103:12-19. doi: 10.1016/j.bone.2017.06.008. Epub 2017 Jun 13. Bone. 2017. PMID: 28627474 Free PMC article.
-
Osteogenic Differentiation of Periosteal Cells During Fracture Healing.J Cell Physiol. 2017 May;232(5):913-921. doi: 10.1002/jcp.25641. Epub 2016 Oct 26. J Cell Physiol. 2017. PMID: 27731505 Free PMC article. Review.
-
Mechanically induced osteogenic lineage commitment of stem cells.Stem Cell Res Ther. 2013;4(5):107. doi: 10.1186/scrt318. Stem Cell Res Ther. 2013. PMID: 24004875 Free PMC article. Review.
Cited by
-
Progress of Periosteal Osteogenesis: The Prospect of In Vivo Bioreactor.Orthop Surg. 2022 Sep;14(9):1930-1939. doi: 10.1111/os.13325. Epub 2022 Jul 6. Orthop Surg. 2022. PMID: 35794789 Free PMC article. Review.
-
Activation of creER recombinase in the mouse calvaria induces local recombination without effects on distant skeletal segments.Sci Rep. 2021 Apr 15;11(1):8214. doi: 10.1038/s41598-021-87611-2. Sci Rep. 2021. PMID: 33859263 Free PMC article.
-
Periosteum progenitors could stimulate bone regeneration in aged murine bone defect model.J Cell Mol Med. 2020 Oct;24(20):12199-12210. doi: 10.1111/jcmm.15891. Epub 2020 Sep 15. J Cell Mol Med. 2020. PMID: 32931157 Free PMC article.
-
Hedgehog signaling is necessary and sufficient to mediate craniofacial plasticity in teleosts.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19321-19327. doi: 10.1073/pnas.1921856117. Epub 2020 Jul 27. Proc Natl Acad Sci U S A. 2020. PMID: 32719137 Free PMC article.
-
Mechanical regulation of bone remodeling.Bone Res. 2022 Feb 18;10(1):16. doi: 10.1038/s41413-022-00190-4. Bone Res. 2022. PMID: 35181672 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

