Urine-derived cells for human cell therapy
- PMID: 29996911
- PMCID: PMC6042455
- DOI: 10.1186/s13287-018-0932-z
Urine-derived cells for human cell therapy
Erratum in
-
Correction to: Urine-derived cells for human cell therapy.Stem Cell Res Ther. 2018 Aug 22;9(1):222. doi: 10.1186/s13287-018-0974-2. Stem Cell Res Ther. 2018. PMID: 30134974 Free PMC article.
Abstract
Desirable cells for human cell therapy would be ones that can be generated by simple isolation and culture techniques using a donor sample obtained by non-invasive methods. To date, the different donor-specific cells that can be isolated from blood, skin, and hair require invasive methods for sample isolation and incorporate complex and costly reagents to culture. These cells also take considerable time for their in-vitro isolation and expansion. Previous studies suggest that donor-derived cells, namely urine stem cells and renal cells, may be isolated from human urine samples using a cost-effective and simple method of isolation, incorporating not such complex reagents. Moreover, the isolated cells, particularly urine stem cells, are superior to conventional stem cell sources in terms of favourable gene profile and inherent multipotent potential. Transdifferentiation or differentiation of human urine-derived cells can generate desirable cells for regenerative therapy. In this review, we intended to discuss the characteristics and therapeutic applications of urine-derived cells for human cell therapy. Conclusively, with detailed study and optimisation, urine-derived cells have a prospective future to generate functional lineage-specific cells for patients from a clinical translation point of view.
Keywords: Differentiation; Renal cells; Stem cells; Therapy; Urine.
Conflict of interest statement
Ethics approval and consent to participate
Human urine collection and storage was performed in accordance with the Ethics committee of University of South Australia (human ethic number 0000035945).
Consent for publication
All authors have approved the data for the submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
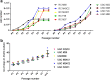

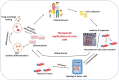
References
-
- Liu G, Deng C, Zhang Y. Urine-derived stem cells: biological characterization and potential clinical applications, Stem Cells: Current Challenges and New Directions, 2013;20:19–28.
-
- Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:5. - PubMed
-
- Kloskowski T, Nowacki M, Pokrywczynska M, Drewa T. Urine—a waste or the future of regenerative medicine? Med Hypotheses. 2015;84:4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

