Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database
- PMID: 29996929
- PMCID: PMC6042444
- DOI: 10.1186/s12895-018-0074-0
Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database
Abstract
Background: Biological therapies (BTs) including infliximab (IFX), adalimumab (ADL), secukinumab (SCK) and ustekinumab (UST) are approved in Japan for the treatment of psoriasis. Although the persistence rates and medical costs of BTs treatment have been investigated in multiple foreign studies in recent years, few such studies have been conducted in Japan and the differences between patients who adhered to treatment and those who did not have not been reported. This study is aimed at investigating the persistence rates and medical costs of BTs in the treatment of psoriasis in Japan, using the real-world data from a large-scale claims database.
Methods: Claims data from the JMDC database (August 2009 to December 2016) were used for this analysis. Patient data were extracted using the ICD10 code for psoriasis and claims records of BT injections. Twelve-month and 24-month persistence rates of BTs were estimated by Kaplan-Meier methodology, and 12-month-medical costs before and after BT initiation were compared between persistent and non-persistent patient groups at 12 months.
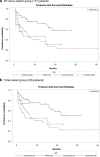
Results: A total of 205 psoriasis patients treated with BTs (BT-naïve patients: 177) were identified. The 12-month/24-month persistence rates for ADL, IFX, SCK, and UST in BT-naïve patients were 46.8% ± 16.6%/46.8 ± 16.6%, 53.0% ± 14.9%/41.0% ± 15.5%, 55.4%/55.4% (95% CI not available) and 79.4% ± 9.9%/71.9% ± 12.2%, respectively. Statistically significant differences in persistence were found among different BT treatments, and UST was found to have the highest persistence rate. The total medical costs during the 12 months after BT initiation in BT-naïve patients were (in 1000 Japanese Yen): 2218 for ADL, 3409 for IFX, 465 for SCK, 2824 for UST (average: 2828). Compared with the 12-month persistent patient group, the total medical costs in the persistent group was higher (Δ:+ 118), but for some medications such as IFX or UST cost increases were lower for persistent patients.
Conclusions: UST was found to have the highest persistence rate among all BTs for psoriasis treatment in Japan. The 12-month medical costs after BT initiation in the persistent patient group may not have increased as much as in the non-persistent patient group for some medications.
Keywords: Biological therapy; Claims database; Medical costs; Persistence; Psoriasis; Real-world data.
Conflict of interest statement
Ethics approval and consent to participate
This was a retrospective study carried out using claims data from the commercial JMDC database; the authors were not involved in the collection of these data and data were anonymized before addition to the research database. Retrieval of the data from this database occurred in an unlinked fashion. As the data had been anonymized, the Ethical Guidelines for Epidemiological Research (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labor and Welfare of Japan), which require ethics approval and informed consent, are not applicable to this study. Based on the Ethical Guidelines on Biomedical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labor and Welfare of Japan), pharmacoepidemiological studies conducted on medical databases qualify as research carried out on pre-existing material and information that do not require any interventions or interactions with patients. For such studies, including this study, obtaining written informed consent from patients is not mandatory. The authors had permission from JMDC to access the database.
Consent for publication
Not applicable.
Competing interests
JM, and RS were affiliated with Janssen Pharmaceutical KK at the time the study was conducted, a company that develops and markets drugs for the treatment of psoriasis. KI and WT received honoraria from Janssen KK.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Drug Survival of Biological Therapies for Psoriasis Treatment in Germany and Associated Costs: A Retrospective Claims Database Analysis.Adv Ther. 2019 Jul;36(7):1684-1699. doi: 10.1007/s12325-019-00969-8. Epub 2019 May 17. Adv Ther. 2019. PMID: 31102203
-
Cost-efficacy comparison of biological therapies for patients with moderate to severe psoriasis in Japan.J Dermatolog Treat. 2013 Oct;24(5):351-5. doi: 10.3109/09546634.2012.697111. Epub 2012 Jul 9. J Dermatolog Treat. 2013. PMID: 22632451 Review.
-
Number needed to treat and costs per responder among biologic treatments for moderate-to-severe plaque psoriasis in Japan.J Dermatolog Treat. 2018 Feb;29(1):24-31. doi: 10.1080/09546634.2017.1341607. Epub 2017 Jun 23. J Dermatolog Treat. 2018. PMID: 28608740
-
Cost-effectiveness of secukinumab as first biologic treatment, compared with other biologics, for moderate to severe psoriasis in Germany.J Eur Acad Dermatol Venereol. 2018 Dec;32(12):2191-2199. doi: 10.1111/jdv.15047. Epub 2018 Jun 27. J Eur Acad Dermatol Venereol. 2018. PMID: 29729105
-
Biologic Therapy for Ulcerative Colitis.Gastroenterol Clin North Am. 2020 Dec;49(4):717-729. doi: 10.1016/j.gtc.2020.08.002. Epub 2020 Sep 26. Gastroenterol Clin North Am. 2020. PMID: 33121691 Review.
Cited by
-
A Retrospective Cohort Analysis of Treatment Patterns Over 1 Year in Patients with Psoriasis Treated with Ixekizumab or Guselkumab.Dermatol Ther (Heidelb). 2022 Mar;12(3):701-714. doi: 10.1007/s13555-022-00686-1. Epub 2022 Feb 26. Dermatol Ther (Heidelb). 2022. PMID: 35220545 Free PMC article.
-
Evaluation of economic burden with biologic treatments in Crohn's disease patients: A mirror image study using an insurance database in Japan.PLoS One. 2021 Jul 19;16(7):e0254807. doi: 10.1371/journal.pone.0254807. eCollection 2021. PLoS One. 2021. PMID: 34280242 Free PMC article.
-
Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: A retrospective claims database study.J Dermatol. 2022 Nov;49(11):1106-1117. doi: 10.1111/1346-8138.16543. Epub 2022 Aug 10. J Dermatol. 2022. PMID: 35946343 Free PMC article.
-
Patient preference for biologic treatments of psoriasis in Japan.J Dermatol. 2019 Jun;46(6):466-477. doi: 10.1111/1346-8138.14870. Epub 2019 Apr 15. J Dermatol. 2019. PMID: 30985030 Free PMC article.
-
Patient and Physician Preferences for Therapy Characteristics for Psoriasis: A Discrete Choice Experiment in Japan.Pharmacoecon Open. 2019 Jun;3(2):255-264. doi: 10.1007/s41669-018-0104-1. Pharmacoecon Open. 2019. PMID: 30377992 Free PMC article.
References
-
- Terui T. Nakagawa H, et al.a survey of the status of psoriasis conducted using information obtained from health insurance claims provided by health insurance societies. J Clin Ther Med (Rinsyo-iyakku) 2014;30(3):279–285.
-
- Zweegers J, van den Reek JM, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice. A prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol. 2016;175(2):340–347. doi: 10.1111/bjd.14552. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

