When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance
- PMID: 29996946
- PMCID: PMC6042406
- DOI: 10.1186/s40880-018-0317-9
When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance
Abstract
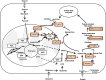
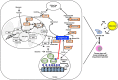
The role of fatty acid metabolism, including both anabolic and catabolic reactions in cancer has gained increasing attention in recent years. Many studies have shown that aberrant expression of the genes involved in fatty acid synthesis or fatty acid oxidation correlate with malignant phenotypes including metastasis, therapeutic resistance and relapse. Such phenotypes are also strongly associated with the presence of a small percentage of unique cells among the total tumor cell population. This distinct group of cells may have the ability to self-renew and propagate or may be able to develop resistance to cancer therapies independent of genetic alterations. Therefore, these cells are referred to as cancer stem cells/tumor-initiating cells/drug-tolerant persisters, which are often refractory to cancer treatment and difficult to target. Moreover, interconversion between cancer cells and cancer stem cells/tumor-initiating cells/drug-tolerant persisters may occur and makes treatment even more challenging. This review highlights recent findings on the relationship between fatty acid metabolism, cancer stemness and therapeutic resistance and prompts discussion about the potential mechanisms by which fatty acid metabolism regulates the fate of cancer cells and therapeutic resistance.
Keywords: Cancer cell plasticity; Cancer stem cells; Drug-tolerant persisters; Fatty acid metabolism; Fatty acid oxidation; Fatty acid synthesis; Lipogenic phenotype; Therapeutic resistance; Tumor-initiating cells.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

