Regulation of Trypanosoma brucei Acetyl Coenzyme A Carboxylase by Environmental Lipids
- PMID: 29997119
- PMCID: PMC6041502
- DOI: 10.1128/mSphere.00164-18
Regulation of Trypanosoma brucei Acetyl Coenzyme A Carboxylase by Environmental Lipids
Abstract
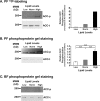
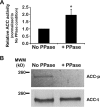
To satisfy its fatty acid needs, the extracellular eukaryotic parasite Trypanosoma brucei relies on two mechanisms: uptake of fatty acids from the host and de novo synthesis. We hypothesized that T. brucei modulates fatty acid synthesis in response to environmental lipid availability. The first committed step in fatty acid synthesis is catalyzed by acetyl coenzyme A (acetyl-CoA) carboxylase (ACC) and serves as a key regulatory point in other organisms. To test our hypothesis, T. brucei mammalian bloodstream and insect procyclic forms were grown in low-, normal-, or high-lipid media and the effect on T. brucei ACC (TbACC) mRNA, protein, and enzymatic activity was examined. In bloodstream form T. brucei, media lipids had no effect on TbACC expression or activity. In procyclic form T. brucei, we detected no change in TbACC mRNA levels but observed 2.7-fold-lower TbACC protein levels and 37% lower TbACC activity in high-lipid media than in low-lipid media. Supplementation of low-lipid media with the fatty acid stearate mimicked the effect of high lipid levels on TbACC activity. In procyclic forms, TbACC phosphorylation also increased 3.9-fold in high-lipid media compared to low-lipid media. Phosphatase treatment of TbACC increased activity, confirming that phosphorylation represented an inhibitory modification. Together, these results demonstrate a procyclic-form-specific environmental lipid response pathway that regulates TbACC posttranscriptionally, through changes in protein expression and phosphorylation. We propose that this environmental response pathway enables procyclic-form T. brucei to monitor the host lipid supply and downregulate fatty acid synthesis when host lipids are abundant and upregulate fatty acid synthesis when host lipids become scarce.IMPORTANCETrypanosoma brucei is a eukaryotic parasite that causes African sleeping sickness. T. brucei is transmitted by the blood-sucking tsetse fly. In order to adapt to its two very different hosts, T. brucei must sense the host environment and alter its metabolism to maximize utilization of host resources and minimize expenditure of its own resources. One key nutrient class is represented by fatty acids, which the parasite can either take from the host or make themselves. Our work describes a novel environmental regulatory pathway for fatty acid synthesis where the parasite turns off fatty acid synthesis when environmental lipids are abundant and turns on synthesis when the lipid supply is scarce. This pathway was observed in the tsetse midgut form but not the mammalian bloodstream form. However, pharmacological activation of this pathway in the bloodstream form to turn fatty acid synthesis off may be a promising new avenue for sleeping sickness drug discovery.
Keywords: Trypanosoma brucei; acetyl-CoA carboxylase; enzyme regulation; fatty acids; lipids; parasitology; protein phosphorylation.
Copyright © 2018 Ray et al.
Figures



References
-
- Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, Bento F, Young SA, Pinto A, Van Den Abbeele J, Ribeiro RM, Dias S, Smith TK, Figueiredo LM. 2016. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe 19:837–848. doi:10.1016/j.chom.2016.05.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

