CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec-10 receptor and suppress human T cell function
- PMID: 29997173
- PMCID: PMC6065011
- DOI: 10.1073/pnas.1722056115
CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec-10 receptor and suppress human T cell function
Erratum in
-
Correction for Bandala-Sanchez et al., CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec-10 receptor and suppress human T cell function.Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7592-7593. doi: 10.1073/pnas.1904079116. Epub 2019 Apr 1. Proc Natl Acad Sci U S A. 2019. PMID: 30936310 Free PMC article. No abstract available.
Abstract
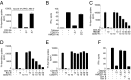
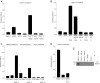
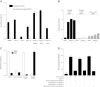
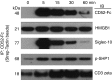
CD52, a glycophosphatidylinositol (GPI)-anchored glycoprotein, is released in a soluble form following T cell activation and binds to the Siglec (sialic acid-binding Ig-like lectin)-10 receptor on T cells to suppress their function. We show that binding of CD52-Fc to Siglec-10 and T cell suppression requires the damage-associated molecular pattern (DAMP) protein, high-mobility group box 1 (HMGB1). CD52-Fc bound specifically to the proinflammatory Box B domain of HMGB1, and this in turn promoted binding of the CD52 N-linked glycan, in α-2,3 sialic acid linkage with galactose, to Siglec-10. Suppression of T cell function was blocked by anti-HMGB1 antibody or the antiinflammatory Box A domain of HMGB1. CD52-Fc induced tyrosine phosphorylation of Siglec-10 and was recovered from T cells complexed with HMGB1 and Siglec-10 in association with SHP1 phosphatase and the T cell receptor (TCR). Thus, soluble CD52 exerts a concerted immunosuppressive effect by first sequestering HMGB1 to nullify its proinflammatory Box B, followed by binding to the inhibitory Siglec-10 receptor, triggering recruitment of SHP1 to the intracellular immunoreceptor tyrosine-based inhibitory motif of Siglec-10 and its interaction with the TCR. This mechanism may contribute to immune-inflammatory homeostasis in pathophysiologic states and underscores the potential of soluble CD52 as a therapeutic agent.
Keywords: CD52; HMGB1; Siglec-10; T cell; sialoglycan.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Xia MQ, Tone M, Packman L, Hale G, Waldmann H. Characterization of the CAMPATH-1 (CDw52) antigen: Biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. Eur J Immunol. 1991;21:1677–1684. - PubMed
-
- Hale G. CD52 (CAMPATH1) J Biol Regul Homeost Agents. 2001;15:386–391. - PubMed
-
- Kirchhoff C, Schröter S. New insights into the origin, structure and role of CD52: A major component of the mammalian sperm glycocalyx. Cells Tissues Organs. 2001;168:93–104. - PubMed
-
- Ito K, Hasegawa A, Komori S, Koyama K. Biochemical property and immunogenicity of mouse male reproductive tract CD52 (mrt-CD52) J Reprod Immunol. 2007;75:32–39. - PubMed
-
- Bandala-Sanchez E, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol. 2013;14:741–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

