Reduced-intensity conditioning for hematopoietic cell transplant for HLH and primary immune deficiencies
- PMID: 29997222
- PMCID: PMC6161764
- DOI: 10.1182/blood-2018-01-828277
Reduced-intensity conditioning for hematopoietic cell transplant for HLH and primary immune deficiencies
Abstract
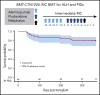
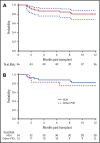
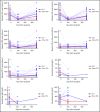
Allogeneic hematopoietic cell transplantation (HCT) with myeloablative conditioning for disorders associated with excessive inflammation such as hemophagocytic lymphohistiocytosis (HLH) is associated with early mortality. A multicenter prospective phase 2 trial of reduced-intensity conditioning with melphalan, fludarabine, and intermediate-timing alemtuzumab was conducted for HLA matched or single HLA locus mismatched related or unrelated donor HCT in a largely pediatric cohort. Graft-versus-host disease (GVHD) prophylaxis was cyclosporine with methylprednisolone. The primary end point was 1-year overall survival (OS). Thirty-four patients with HLH and 12 with other primary immune deficiencies were transplanted. With a median follow-up of 20 months, the 1-year OS for transplanted patients was 80.4% (90% confidence interval [CI], 68.6%-88.2%). Five additional deaths by 16 months yielded an 18-month OS probability of 66.7% (90% CI, 52.9%-77.3%). Two patients experienced primary graft failure, and 18 patients either experienced a secondary graft failure or required a second intervention (mostly donor lymphocyte infusion [DLI]). At 1 year, the proportion of patients alive with sustained engraftment without DLI or second HCT was 39.1% (95% CI, 25.2%-54.6%), and that of being alive and engrafted (with or without DLI) was 60.9% (95% CI, 45.4 %-74.9%). The day 100 incidence of grade II to IV acute GVHD was 17.4% (95% CI, 8.1%-29.7%), and 1-year incidence of chronic GVHD was 26.7% (95% CI, 14.6%-40.4%). Although the trial demonstrated low early mortality, the majority of surviving patients required DLI or second HCT. These results demonstrate a need for future approaches that maintain low early mortality with improved sustained engraftment. The trial was registered at Clinical Trials.gov (NCT 01998633).
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Comment in
-
Finding "intermediate" ground in transplant and HLH.Blood. 2018 Sep 27;132(13):1361-1363. doi: 10.1182/blood-2018-08-870014. Blood. 2018. PMID: 30262583 No abstract available.
References
-
- Horne A, Trottestam H, Aricò M, et al. ; Histiocyte Society. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140(3):327-335. - PubMed
-
- Ouachée-Chardin M, Elie C, de Saint Basile G, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006;117(4):e743-e750. - PubMed
-
- Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42(3):175-180. - PubMed
-
- Cesaro S, Locatelli F, Lanino E, et al. Hematopoietic stem cell transplantation for hemophagocytic lymphohistiocytosis: a retrospective analysis of data from the Italian Association of Pediatric Hematology Oncology (AIEOP). Haematologica. 2008;93(11):1694-1701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

