Interorganelle Communication: Peroxisomal MALATE DEHYDROGENASE2 Connects Lipid Catabolism to Photosynthesis through Redox Coupling in Chlamydomonas
- PMID: 29997239
- PMCID: PMC6139685
- DOI: 10.1105/tpc.18.00361
Interorganelle Communication: Peroxisomal MALATE DEHYDROGENASE2 Connects Lipid Catabolism to Photosynthesis through Redox Coupling in Chlamydomonas
Abstract
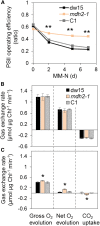
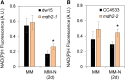
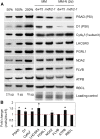
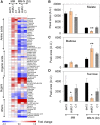
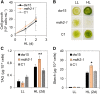
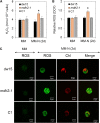
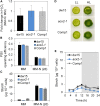
Plants and algae must tightly coordinate photosynthetic electron transport and metabolic activities given that they often face fluctuating light and nutrient conditions. The exchange of metabolites and signaling molecules between organelles is thought to be central to this regulation but evidence for this is still fragmentary. Here, we show that knocking out the peroxisome-located MALATE DEHYDROGENASE2 (MDH2) of Chlamydomonas reinhardtii results in dramatic alterations not only in peroxisomal fatty acid breakdown but also in chloroplast starch metabolism and photosynthesis. mdh2 mutants accumulated 50% more storage lipid and 2-fold more starch than the wild type during nitrogen deprivation. In parallel, mdh2 showed increased photosystem II yield and photosynthetic CO2 fixation. Metabolite analyses revealed a >60% reduction in malate, together with increased levels of NADPH and H2O2 in mdh2 Similar phenotypes were found upon high light exposure. Furthermore, based on the lack of starch accumulation in a knockout mutant of the H2O2-producing peroxisomal ACYL-COA OXIDASE2 and on the effects of H2O2 supplementation, we propose that peroxisome-derived H2O2 acts as a regulator of chloroplast metabolism. We conclude that peroxisomal MDH2 helps photoautotrophs cope with nitrogen scarcity and high light by transmitting the redox state of the peroxisome to the chloroplast by means of malate shuttle- and H2O2-based redox signaling.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures










Similar articles
-
Subcellular Energetics and Carbon Storage in Chlamydomonas.Cells. 2019 Sep 26;8(10):1154. doi: 10.3390/cells8101154. Cells. 2019. PMID: 31561610 Free PMC article. Review.
-
Chlamydomonas carries out fatty acid β-oxidation in ancestral peroxisomes using a bona fide acyl-CoA oxidase.Plant J. 2017 Apr;90(2):358-371. doi: 10.1111/tpj.13498. Epub 2017 Mar 20. Plant J. 2017. PMID: 28142200
-
Metabolic and photosynthetic consequences of blocking starch biosynthesis in the green alga Chlamydomonas reinhardtii sta6 mutant.Plant J. 2015 Mar;81(6):947-60. doi: 10.1111/tpj.12783. Plant J. 2015. PMID: 25645872
-
Putative role of the malate valve enzyme NADP-malate dehydrogenase in H2O2 signalling in Arabidopsis.Philos Trans R Soc Lond B Biol Sci. 2014 Mar 3;369(1640):20130228. doi: 10.1098/rstb.2013.0228. Print 2014 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24591715 Free PMC article.
-
Chloroplast redox signals: how photosynthesis controls its own genes.Trends Plant Sci. 2003 Jan;8(1):33-41. doi: 10.1016/s1360-1385(02)00005-5. Trends Plant Sci. 2003. PMID: 12523998 Review.
Cited by
-
Iron rescues glucose-mediated photosynthesis repression during lipid accumulation in the green alga Chromochloris zofingiensis.Nat Commun. 2024 Jul 18;15(1):6046. doi: 10.1038/s41467-024-50170-x. Nat Commun. 2024. PMID: 39025848 Free PMC article.
-
Subcellular Energetics and Carbon Storage in Chlamydomonas.Cells. 2019 Sep 26;8(10):1154. doi: 10.3390/cells8101154. Cells. 2019. PMID: 31561610 Free PMC article. Review.
-
CrABCA2 Facilitates Triacylglycerol Accumulation in Chlamydomonas reinhardtii under Nitrogen Starvation.Mol Cells. 2020 Jan 31;43(1):48-57. doi: 10.14348/molcells.2019.0262. Mol Cells. 2020. PMID: 31910336 Free PMC article.
-
Co-Expression of Lipid Transporters Simultaneously Enhances Oil and Starch Accumulation in the Green Microalga Chlamydomonas reinhardtii under Nitrogen Starvation.Metabolites. 2023 Jan 10;13(1):115. doi: 10.3390/metabo13010115. Metabolites. 2023. PMID: 36677040 Free PMC article.
-
The green algae CO2 concentrating mechanism and photorespiration jointly operate during acclimation to low CO2.Nat Commun. 2025 Jun 17;16(1):5296. doi: 10.1038/s41467-025-60525-7. Nat Commun. 2025. PMID: 40527898 Free PMC article.
References
-
- Aboelmy M.H., Peterhansel C. (2014). Enzymatic characterization of Chlamydomonas reinhardtii glycolate dehydrogenase and its nearest proteobacterial homologue. Plant Physiol. Biochem. 79: 25–30. - PubMed
-
- Araújo W.L., Tohge T., Osorio S., Lohse M., Balbo I., Krahnert I., Sienkiewicz-Porzucek A., Usadel B., Nunes-Nesi A., Fernie A.R. (2012). Antisense inhibition of the 2-oxoglutarate dehydrogenase complex in tomato demonstrates its importance for plant respiration and during leaf senescence and fruit maturation. Plant Cell 24: 2328–2351. - PMC - PubMed
-
- Bailleul B., et al. (2015). Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524: 366–369. - PubMed
-
- Ball S., Dirick L., Decq A., Martiat J., Matagne R. (1990). Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci. 66: 1–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

