Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis
- PMID: 29997288
- PMCID: PMC6124519
- DOI: 10.1172/jci.insight.95319
Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis
Abstract
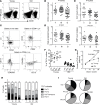
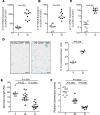
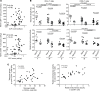
The maintenance of effective immunity over time is dependent on the capacity of hematopoietic stem cells (HSCs) to sustain the pool of immunocompetent mature cells. Decline of immune competence with old age may stem from HSC defects, including reduced self-renewal potential and impaired lymphopoiesis, as suggested in murine models. To obtain further insights into aging-related alteration of hematopoiesis, we performed a comprehensive study of blood hematopoietic progenitor cells (HPCs) from older humans. In the elderly, HPCs present active oxidative phosphorylation and are pressed to enter cell cycling. However, p53-p21 and p15 cell senescence pathways, associated with telomerase activity deficiency, strong telomere attrition, and oxidative stress, are engaged, thus limiting cell cycling. Moreover, survival of old HPCs is impacted by pyroptosis, an inflammatory form of programmed cell death. Lastly, telomerase activity deficiency and telomere length attrition of old HPCs may be passed on to progeny cells such as naive T lymphocytes, further highlighting the poor hematopoietic potential of the elderly. This pre-senescent profile is characteristic of the multiple intrinsic and extrinsic factors affecting HPCs in elderly individuals and represents a major obstacle in terms of immune reconstitution and efficacy with advanced age.
Keywords: Aging; Cellular senescence; Hematopoietic stem cells; Human stem cells; Stem cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

