Spatial and temporal variations in hemodynamic forces initiate cardiac trabeculation
- PMID: 29997298
- PMCID: PMC6124527
- DOI: 10.1172/jci.insight.96672
Spatial and temporal variations in hemodynamic forces initiate cardiac trabeculation
Abstract
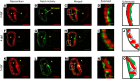
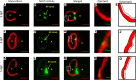
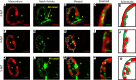
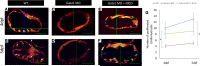
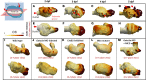
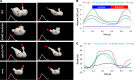
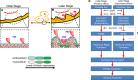
Hemodynamic shear force has been implicated as modulating Notch signaling-mediated cardiac trabeculation. Whether the spatiotemporal variations in wall shear stress (WSS) coordinate the initiation of trabeculation to influence ventricular contractile function remains unknown. Using light-sheet fluorescent microscopy, we reconstructed the 4D moving domain and applied computational fluid dynamics to quantify 4D WSS along the trabecular ridges and in the groves. In WT zebrafish, pulsatile shear stress developed along the trabecular ridges, with prominent endocardial Notch activity at 3 days after fertilization (dpf), and oscillatory shear stress developed in the trabecular grooves, with epicardial Notch activity at 4 dpf. Genetic manipulations were performed to reduce hematopoiesis and inhibit atrial contraction to lower WSS in synchrony with attenuation of oscillatory shear index (OSI) during ventricular development. γ-Secretase inhibitor of Notch intracellular domain (NICD) abrogated endocardial and epicardial Notch activity. Rescue with NICD mRNA restored Notch activity sequentially from the endocardium to trabecular grooves, which was corroborated by observed Notch-mediated cardiomyocyte proliferations on WT zebrafish trabeculae. We also demonstrated in vitro that a high OSI value correlated with upregulated endothelial Notch-related mRNA expression. In silico computation of energy dissipation further supports the role of trabeculation to preserve ventricular structure and contractile function. Thus, spatiotemporal variations in WSS coordinate trabecular organization for ventricular contractile function.
Keywords: Cardiology; Development; Embryonic development; Heart failure.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

