Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing
- PMID: 29997302
- PMCID: PMC6124521
- DOI: 10.1172/jci.insight.120546
Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing
Erratum in
-
Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing.JCI Insight. 2025 Oct 8;10(20):e198650. doi: 10.1172/jci.insight.198650. eCollection 2025 Oct 8. JCI Insight. 2025. PMID: 40985887 Free PMC article. No abstract available.
Abstract
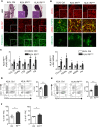
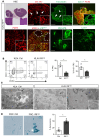
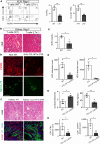
The contribution of the kidney-draining lymph node (KLN) to the pathogenesis of ischemia-reperfusion injury (IRI) of the kidney and its subsequent recovery has not been explored in depth. In addition, the mechanism by which repetitive IRI contributes to renal fibrosis remains poorly understood. Herein, we have found that IRI of the kidney is associated with expansion of high endothelial venules (HEVs) and activation of fibroblastic reticular cells (FRCs) in the KLN, as demonstrated by significant expansion in the extracellular matrix. The lymphotoxin α signaling pathway mediates activation of FRCs, and chronic treatment with lymphotoxin β receptor-immunoglobulin fusion protein (LTβr-Ig) resulted in marked alteration of the KLN as well as augmentation of renal fibrosis. Depletion of FRCs reduced T cell activation in the KLN and ameliorated renal injury in acute IRI. Repetitive renal IRI was associated with senescence of FRCs, fibrosis of the KLN, and renal scarring, which were ameliorated by FRC administration. Therefore, our study emphasizes the critical role of FRCs in both the initiation and repair phases of injury following IRI of the kidney.
Keywords: Chronic kidney disease; Fibrosis; Nephrology.
Conflict of interest statement
Figures


Comment in
-
Lymph node fibrosis after ischaemic injury.Nat Rev Nephrol. 2018 Oct;14(10):599. doi: 10.1038/s41581-018-0050-2. Nat Rev Nephrol. 2018. PMID: 30065257 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

