Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading
- PMID: 29997383
- PMCID: PMC6161286
- DOI: 10.3390/membranes8030042
Performance of PES/LSMM-OGCN Photocatalytic Membrane for Phenol Removal: Effect of OGCN Loading
Abstract
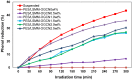
In designing a photocatalytic oxidation system, the immobilized photocatalyst technique becomes highly profitable due to its promising capability in treating organic pollutants such as phenols in wastewater. In this study, hydrophiLic surface modifying macromolecules (LSMM) modified polyethersulfone (PES) hybrid photocatalytic membranes incorporated with oxygenated graphitic carbon nitride (OGCN) was successfully developed using phase inversion technique. The effectiveness of the hybrid photocatalytic membrane was determined under different loading of OGCN photocatalyst (0, 0.5, 1.0, 1.5, 2.0, and 2.5 wt%). The best amount of OGCN in the casting solution was 1.0 wt% as the agglomeration did not occur considering the stability of the membrane performance and morphology. The highest flux of 264 L/m²·h was achieved by PES/LSMM-OGCN1.5wt% membrane. However, the highest flux performance was not an advantage in this situation as the flux reduced the rejection value due to open pores. The membrane with the highest photocatalytic performance was obtained at 1.0 wt% of OGCN loading with 35.78% phenol degradation after 6 h. Regardless of the lower rejection value, the performance shown by the PES/LSMM-OGCN1.0wt% membrane was still competent because of the small difference of less than 1% to that of the PES/LSMM-OGCN0wt% membrane. Based on the findings, it can be concluded that the optimisation of the OGCN loading in the PES hybrid photocatalytic membrane indeed plays an important role towards enhancing the catalyst distribution, phenol degradation, and acceptable rejection above all considerations.
Keywords: hybrid membrane; hydrophilic surface modifying macromolecules; oxygen-doped graphitic carbon nitride; phenol; photocatalytic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Sanders D.F., Smith Z.P., Guo R., Robeson L.M., McGrath J.E., Paul D.R., Freeman B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer. 2013;54:4729–4761. doi: 10.1016/j.polymer.2013.05.075. - DOI
-
- Le N.L., Nunes S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016;7:1–28. doi: 10.1016/j.susmat.2016.02.001. - DOI
-
- Bodalo A., Gomez J.L., Gomez M., Leon G., Hidalgo A.M., Ruiz M.A. Phenol removal from water by hybrid processes: Study of the membrane process step. Desalination. 2008;223:323–329. doi: 10.1016/j.desal.2007.01.219. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

