Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections
- PMID: 29997579
- PMCID: PMC6030385
- DOI: 10.3389/fmicb.2018.01299
Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections
Abstract
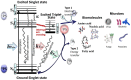
Biofilm describes a microbially-derived sessile community in which microbial cells are firmly attached to the substratum and embedded in extracellular polymeric matrix. Microbial biofilms account for up to 80% of all bacterial and fungal infections in humans. Biofilm-associated pathogens are particularly resistant to antibiotic treatment, and thus novel antibiofilm approaches needed to be developed. Antimicrobial Photodynamic therapy (aPDT) had been recently proposed to combat clinically relevant biofilms such as dental biofilms, ventilator associated pneumonia, chronic wound infections, oral candidiasis, and chronic rhinosinusitis. aPDT uses non-toxic dyes called photosensitizers (PS), which can be excited by harmless visible light to produce reactive oxygen species (ROS). aPDT is a multi-stage process including topical PS administration, light irradiation, and interaction of the excited state with ambient oxygen. Numerous in vitro and in vivo aPDT studies have demonstrated biofilm-eradication or substantial reduction. ROS are produced upon photo-activation and attack adjacent targets, including proteins, lipids, and nucleic acids present within the biofilm matrix, on the cell surface and inside the microbial cells. Damage to non-specific targets leads to the destruction of both planktonic cells and biofilms. The review aims to summarize the progress of aPDT in destroying biofilms and the mechanisms mediated by ROS. Finally, a brief section provides suggestions for future research.
Keywords: biofilm-related infections; microbial biofilms; photochemical mechanisms; photodynamic therapy; photosensitizer structure; reactive oxygen species.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

