Substitution of Mannan-Binding Lectin (MBL)-Deficient Serum With Recombinant MBL Results in the Formation of New MBL/MBL-Associated Serine Protease Complexes
- PMID: 29997613
- PMCID: PMC6030254
- DOI: 10.3389/fimmu.2018.01406
Substitution of Mannan-Binding Lectin (MBL)-Deficient Serum With Recombinant MBL Results in the Formation of New MBL/MBL-Associated Serine Protease Complexes
Abstract
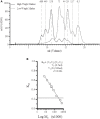
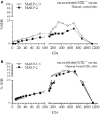
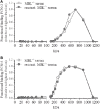
The lectin pathway (LP) of complement activation depends on the activation of the MBL-associated serine proteases (MASPs) circulating in complex with mannan-binding lectin (MBL). MBL deficiency is the most common complement deficiency and has been associated with several pathological conditions. As we had previously shown, plasma-derived MBL (pdMBL) contains pre-activated MASPs that upon in vivo pdMBL substitution results in restoration of MBL concentrations but no LP functionality due to immediate inactivation of pdMBL-MASP complexes upon infusion. In this study, we analyzed MBL-sufficient and -deficient serum by size-exclusion chromatography for complexes of LP activation. In both sera, we identified non-bound free forms of MASP-2 and to lesser extent MASP-1/3. After addition of recombinant MBL (rMBL) to MBL-deficient serum, these free MASPs were much less abundantly present, which is highly suggestive for the formation of high-molecular complexes that could still become activated upon subsequent ligand binding as shown by a restoration of C4-deposition of MBL-deficient serum. Ficolin (FCN)-associated MASPs have been described to redistribute to ligand-bound MBL, hereby forming new MBL/MASP complexes. However, reconstitution of MBL-deficient serum with rMBL did not change the relative size of the FCN molecules suggestive for a limited redistribution in fluid phase of already formed complexes. Our findings demonstrate that rMBL can associate with free non-bound MASPs in fluid phase while preserving full restoration of LP functionality. In contrast to pdMBL products containing pre-activated MASPs which become inactivated almost immediately, these current data provide a rationale for substitution studies using rMBL instead.
Keywords: MBL-associated serine protease; mannan-binding lectin–MBL-associated serine protease complexes; recombinant MBL; redistribution; size-exclusion chromatography.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

