New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue
- PMID: 29997617
- PMCID: PMC6028521
- DOI: 10.3389/fimmu.2018.01432
New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue
Abstract
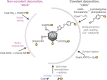
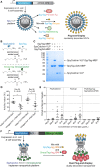
Vaccines based on virus-like particles (VLPs) can induce potent B cell responses. Some non-chimeric VLP-based vaccines are highly successful licensed products (e.g., hepatitis B surface antigen VLPs as a hepatitis B virus vaccine). Chimeric VLPs are designed to take advantage of the VLP framework by decorating the VLP with a different antigen. Despite decades of effort, there have been few licensed chimeric VLP vaccines. Classic approaches to create chimeric VLPs are either genetic fusion or chemical conjugation, using cross-linkers from lysine on the VLP to cysteine on the antigen. We describe the principles that make these classic approaches challenging, in particular for complex, full-length antigens bearing multiple post-translational modifications. We then review recent advances in conjugation approaches for protein-based non-enveloped VLPs or nanoparticles, to overcome such challenges. This includes the use of strong non-covalent assembly methods (stick), unnatural amino acids for bio-orthogonal chemistry (click), and spontaneous isopeptide bond formation by SpyTag/SpyCatcher (glue). Existing applications of these methods are outlined and we critically consider the key practical issues, with particular insight on Tag/Catcher plug-and-display decoration. Finally, we highlight the potential for modular particle decoration to accelerate vaccine generation and prepare for pandemic threats in human and veterinary realms.
Keywords: SpyCatcher; bioconjugation; click chemistry; malaria; synthetic biology; vaccinology; virus-like particle.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

