Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents
- PMID: 29997797
- PMCID: PMC6005136
- DOI: 10.1039/c5sc04572j
Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents
Abstract
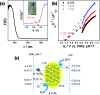
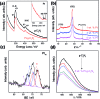
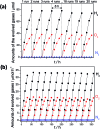
We report the direct splitting of pure water by light-excited graphitic carbon nitride (g-C3N4) modified with Pt, PtO x , and CoO x as redox cocatalysts, while pure g-C3N4 is virtually inactive for overall water splitting by photocatalysis. The novelty is in the selective creation of both H2 and O2 cocatalysts on surface active sites of g-C3N4via photodeposition triggering the splitting of water for the simultaneous evolution of H2 and O2 gases in a stoichiometric ratio of 2 : 1, irradiated with light, without using any sacrificial reagents. The photocatalyst was stable for 510 hours of reaction.
Figures

References
-
- Fujishima A., Honda K. Nature. 1972;238:37–38. - PubMed
-
- Maeda K., Teramura K., Lu D., Takata T., Saito N., Inoue Y., Domen K. Nature. 2006;440:295. - PubMed
-
- Jacobson M., Colella W., Golden D. Science. 2005;308:1901–1905. - PubMed
-
- Yang H., Sun C., Qiao S., Zou J., Liu G., Smith S., Cheng H., Lu G. Nature. 2008;453:638–641. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

