Peroral Low-Dose Toxoplasma gondii Infection of Human Microbiota-Associated Mice - A Subacute Ileitis Model to Unravel Pathogen-Host Interactions
- PMID: 29997912
- PMCID: PMC6038537
- DOI: 10.1556/1886.2018.00005
Peroral Low-Dose Toxoplasma gondii Infection of Human Microbiota-Associated Mice - A Subacute Ileitis Model to Unravel Pathogen-Host Interactions
Abstract
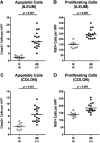
Within 1 week following high-dose Toxoplasma gondii infection, mice develop lethal necrotizing ileitis. However, data from a subacute T. gondii-induced ileitis model are scarce. Therefore, mice harboring a human gut microbiota were perorally infected with one cyst of T. gondii. Within 9 days post-infection, the intestinal microbiota composition shifted towards higher loads of commensal enterobacteria and enterococci. Following T. gondii infection, mice were clinically only mildly affected, whereas ≈60% of mice displayed fecal blood and mild-to-moderate ileal histopathological changes. Intestinal inflammation was further characterized by increased apoptotic intestinal epithelial cells, which were accompanied by elevated proliferating gut epithelial cell numbers. As compared to naive controls, infected mice displayed elevated numbers of intestinal T lymphocytes and regulatory T-cells and increased pro-inflammatory mediator secretion. Remarkably, T. gondii-induced apoptotic and pro-inflammatory immune responses were not restricted to the gut, but could also be observed in extra-intestinal compartments including kidney, liver, and lung. Strikingly, low-dose T. gondii infection resulted in increased serum levels of pro- and anti-inflammatory cytokines. In conclusion, the here presented subacute ileitis model following peroral low-dose T. gondii infection of humanized mice allows for detailed investigations of the molecular mechanism underlying the "ménage à trois" of pathogens, human gut microbiota, and immunity.
Keywords: Toxoplasma gondii; extra-intestinal; fecal microbiota transplantation; host immunity; host-pathogen interactions; human microbiota; intestinal; secondary abiotic mice; subacute ileitis mouse model; systemic immune responses.
Figures










References
-
- McLeod R, Skamene E, Brown CR, Eisenhauer PB, Mack DG. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of A x B/B x A recombinant inbred and B10 congenic mice. J Immunol. 1989;143:3031–4. - PubMed
-
- McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989;142:3247–55. - PubMed
-
- Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–85. - PubMed
-
- Liesenfeld O, Kang H, Park D, Nguyen TA, Parkhe CV, Watanabe H, et al. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
