NOTCH1 Aberrations in Chronic Lymphocytic Leukemia
- PMID: 29998084
- PMCID: PMC6030253
- DOI: 10.3389/fonc.2018.00229
NOTCH1 Aberrations in Chronic Lymphocytic Leukemia
Abstract
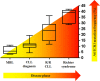
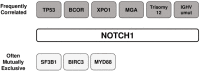
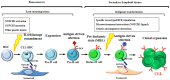
Chronic lymphocytic leukemia (CLL) is an incurable B-cell neoplasm characterized by highly variable clinical outcomes. In recent years, genomic and molecular studies revealed a remarkable heterogeneity in CLL, which mirrored the clinical diversity of this disease. These studies profoundly enhanced our understanding of leukemia cell biology and led to the identification of new biomarkers with potential prognostic and therapeutic significance. Accumulating evidence indicates a key role of deregulated NOTCH1 signaling and NOTCH1 mutations in CLL. This review highlights recent discoveries that improve our understanding of the pathophysiological NOTCH1 signaling in CLL and the clinical impact of NOTCH1 mutations in retrospective and prospective trials. In addition, we discuss the rationale for a therapeutic strategy aiming at inhibiting NOTCH1 signaling in CLL, along with an overview on the currently available NOTCH1-directed approaches.
Keywords: NOTCH1; chronic lymphocytic leukemia; gene mutation; prognostic biomarker; targeted therapy.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

