New Insights Into DNA Helicases as Druggable Targets for Cancer Therapy
- PMID: 29998112
- PMCID: PMC6028597
- DOI: 10.3389/fmolb.2018.00059
New Insights Into DNA Helicases as Druggable Targets for Cancer Therapy
Abstract
Small molecules that deter the functions of DNA damage response machinery are postulated to be useful for enhancing the DNA damaging effects of chemotherapy or ionizing radiation treatments to combat cancer by impairing the proliferative capacity of rapidly dividing cells that accumulate replicative lesions. Chemically induced or genetic synthetic lethality is a promising area in personalized medicine, but it remains to be optimized. A new target in cancer therapy is DNA unwinding enzymes known as helicases. Helicases play critical roles in all aspects of nucleic acid metabolism. We and others have investigated small molecule targeted inhibition of helicase function by compound screens using biochemical and cell-based approaches. Small molecule-induced trapping of DNA helicases may represent a generalized mechanism exemplified by certain topoisomerase and PARP inhibitors that exert poisonous consequences, especially in rapidly dividing cancer cells. Taking the lead from the broader field of DNA repair inhibitors and new information gleaned from structural and biochemical studies of DNA helicases, we predict that an emerging strategy to identify useful helicase-interacting compounds will be structure-based molecular docking interfaced with a computational approach. Potency, specificity, drug resistance, and bioavailability of helicase inhibitor drugs and targeting such compounds to subcellular compartments where the respective helicases operate must be addressed. Beyond cancer therapy, continued and new developments in this area may lead to the discovery of helicase-interacting compounds that chemically rescue clinically relevant helicase missense mutant proteins or activate the catalytic function of wild-type DNA helicases, which may have novel therapeutic application.
Keywords: DNA repair; cancer; genomic instability; helicase; replication; small molecule; therapy.
Figures

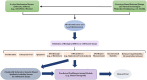
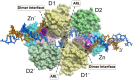



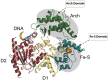
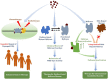
References
-
- Aggarwal M., Banerjee T., Sommers J. A., Iannascoli C., Pichierri P., Shoemaker R. H., et al. . (2013b). Werner syndrome helicase has a critical role in DNA damage responses in the absence of a functional fanconi anemia pathway. Cancer Res. 73, 5497–5507. 10.1158/0008-5472.can-12-2975 - DOI - PMC - PubMed
-
- Aggarwal M., Sommers J. A., Shoemaker R. H., Brosh R. M., Jr. (2011). Inhibition of helicase activity by a small molecule impairs Werner syndrome helicase (WRN) function in the cellular response to DNA damage or replication stress. Proc. Natl. Acad. Sci. U.S.A. 108, 1525–1530. 10.1073/pnas.1006423108 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

