Preclinical Development of Oncolytic Immunovirotherapy for Treatment of HPVPOS Cancers
- PMID: 29998190
- PMCID: PMC6037044
- DOI: 10.1016/j.omto.2018.05.001
Preclinical Development of Oncolytic Immunovirotherapy for Treatment of HPVPOS Cancers
Abstract
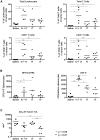
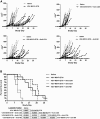
Immunotherapy for HPVPOS malignancies is attractive because well-defined, viral, non-self tumor antigens exist as targets. Several approaches to vaccinate therapeutically against HPV E6 and E7 antigens have been adopted, including viral platforms such as VSV. A major advantage of VSV expressing these antigens is that VSV also acts as an oncolytic virus, leading to direct tumor cell killing and induction of effective anti-E6 and anti-E7 T cell responses. We have also shown that addition of immune adjuvant genes, such as IFNβ, further enhances safety and/or efficacy of VSV-based oncolytic immunovirotherapies. However, multiple designs of the viral vector are possible-with respect to levels of immunogen expression and method of virus attenuation-and optimal designs have not previously been tested head-to-head. Here, we tested three different VSV engineered to express a non-oncogenic HPV16 E7/6 fusion protein for their immunotherapeutic and oncolytic properties. We assessed their profiles of efficacy and toxicity against HPVPOS and HPVNEG murine tumor models and determined the optimal route of administration. Our data show that VSV is an excellent platform for the oncolytic immunovirotherapy of tumors expressing HPV target antigens, combining a balance of efficacy and safety suitable for evaluation in a first-in-human clinical trial.
Keywords: HPV positive cancer; VSV immunovirotherapy; preclinical.
Figures






References
-
- Ott P.A., Piha-Paul S.A., Munster P., Pishvaian M.J., van Brummelen E.M.J., Cohen R.B., Gomez-Roca C., Ejadi S., Stein M., Chan E. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017;28:1036–1041. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

