AftD functions as an α1 → 5 arabinofuranosyltransferase involved in the biosynthesis of the mycobacterial cell wall core
- PMID: 29998212
- PMCID: PMC6034362
- DOI: 10.1016/j.tcsw.2017.10.001
AftD functions as an α1 → 5 arabinofuranosyltransferase involved in the biosynthesis of the mycobacterial cell wall core
Abstract
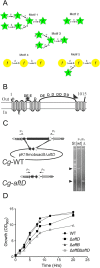
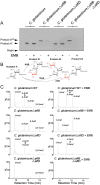
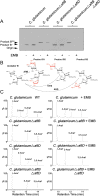
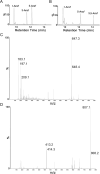
Arabinogalactan (AG) is an essential structural macromolecule present in the cell wall of Mycobacterium tuberculosis, serving to connect peptidoglycan with the outer mycolic acid layer. The D-arabinan segment is a highly branched component of AG and is assembled in a step-wise fashion by a variety of arabinofuranosyltransferases (AraT). We have previously used Corynebacterium glutamicum as a model organism to study these complex processes which are otherwise essential in mycobacteria. In order to further our understanding of the molecular basis of AG assembly, we investigated the role of a fourth AraT, now termed AftD by generating single (ΔaftD) and double deletion (ΔaftB ΔaftD) mutants of C. glutamicum. We demonstrate that AftD functions as an α(1 → 5) AraT and reveal the point at which it exerts its activity in the AG biosynthetic pathway.
Keywords: Arabinogalactan; Cell wall; Corynebacterium glutamicum; Glycosyltransferase; Mycobacterium tuberculosis.
Figures

References
-
- Abbott D.W., Hrynuik S., Boraston A.B. Identification and characterization of a novel periplasmic polygalacturonic acid binding protein from Yersinia enterolitica. J. Mol. Biol. 2007;367:1023–1033. - PubMed
-
- Alderwick L.J., Dover L.G., Seidel M., Gande R., Sahm H., Eggeling L., Besra G.S. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-D-arabinose metabolism. Glycobiology. 2006;16:1073–1081. - PubMed
-
- Alderwick L.J., Radmacher E., Seidel M., Gande R., Hitchen P.G., Morris H.R., Dell A., Sahm H., Eggeling L., Besra G.S. Deletion of Cg-emb in corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J. Biol. Chem. 2005;280:32362–32371. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

