Bispecific antibodies in cancer immunotherapy
- PMID: 29998217
- PMCID: PMC5933537
- DOI: 10.1177/2515135518763280
Bispecific antibodies in cancer immunotherapy
Abstract
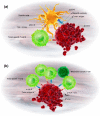
Following the clinical success of immune checkpoint antibodies targeting CTLA-4, PD-1 or PD-L1 in cancer treatment, bispecific antibodies are now emerging as a growing class of immunotherapies with potential to further improve clinical efficacy and safety. We describe three classes of immunotherapeutic bispecific antibodies: (a) cytotoxic effector cell redirectors; (b) tumor-targeted immunomodulators; and (c) dual immunomodulators. Cytotoxic effector cell redirectors are dominated by T-cell redirecting compounds, bispecific compounds engaging a tumor-associated antigen and the T-cell receptor/CD3 complex, thereby redirecting T-cell cytotoxicity to malignant cells. This is the most established class of bispecific immunotherapies, with two compounds having reached the market and numerous compounds in clinical development. Tumor-targeted immunomodulators are bispecific compounds binding to a tumor-associated antigen and an immunomodulating receptor, such as CD40 or 4-1BB. Such compounds are usually designed to be inactive until binding the tumor antigen, thereby localizing immune stimulation to the tumor environment, while minimizing immune activation elsewhere. This is expected to induce powerful activation of tumor-specific T cells with reduced risk of immune-related adverse events. Finally, dual immunomodulators are bispecific compounds that bind two distinct immunomodulating targets, often combining targeting of PD-1 or PD-L1 with that of LAG-3 or TIM-3. The rationale is to induce superior tumor immunity compared to monospecific antibodies to the same targets. In this review, we describe each of these classes of bispecific antibodies, and present examples of compounds in development.
Keywords: bispecific antibody; cancer; checkpoint; co-stimulation; immuno-oncology; immunotherapy.
Conflict of interest statement
Conflict of interest statement: The authors are employees of Alligator Bioscience, a company developing mono- and bispecific antibodies in cancer immunotherapy.
Figures


References
-
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016; 27: 559–574. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

