223Ra-Dichloride in castration-resistant metastatic prostate cancer: improving outcomes and identifying predictors of survival in clinical practice
- PMID: 29998419
- PMCID: PMC6208810
- DOI: 10.1007/s00259-018-4083-3
223Ra-Dichloride in castration-resistant metastatic prostate cancer: improving outcomes and identifying predictors of survival in clinical practice
Abstract
Purpose: We first assessed whether the pattern of referrals to a nuclear medicine clinic improved as experience with 223Ra-dichloride increased, and whether referral patterns affected patient outcomes, and second assessed the value of bone scintigraphy, total alkaline phosphatase (tALP) and lymphadenopathy as prognostic factors in patients receiving 223Ra-dichloride.
Methods: A total of 57 patients eligible to receive 223Ra-dichloride over a 2-year period (March 2014 to March 2016) were retrospectively assessed and prospectively followed (median follow up 298 days). 223Ra-Dichloride was administered at 4-week intervals for a maximum of six injections. The numbers of patients in years 1 and 2 referred in relation to extent of bone disease (EOBD) category and overall survival (OS) were determined. The prognostic factors EOBD category, baseline tALP (tALPBL), tALP response, greatest percentage reduction in tALP from baseline in any treatment cycle (ALPmax; among patients with elevated ALPBL), and the presence of lymphadenopathy were assessed as predictors of OS.
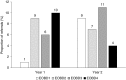
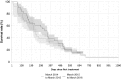
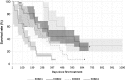
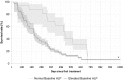
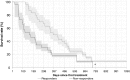
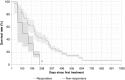
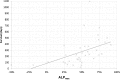
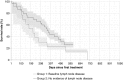
Results: The proportion of patients with EOBD1 was higher in year 2 than in year 1 (29% and 4%, respectively), and in year 2 there was a lower rate of symptomatic skeleton-related events, a higher proportion of patients completing six cycles, and longer (albeit nonsignificant) OS (p = 0.55). There were significant differences in OS between EOBD4 patients and those in all other groups and between EOBD1 and EOBD3 patients (p < 0.05). OS was longer in patients with normal tALPBL than in those with elevated tALPBL (p = 0.01), in ALP responders than in nonresponders (p < 0.05), and in patients without lymphadenopathy than in those with lymphadenopathy (p = 0.29). OS was correlated with ALPmax (r2 = 0.24).
Conclusion: A collaborative multidisciplinary referrals pathway, together with increased experience with 223Ra-dichloride, led to improved outcomes. In patients with elevated tALPBL, tALP dynamics may be useful for monitoring response and predicting OS. Imaging and prognostic markers may therefore be of value for individualizing 223Ra-dichloride treatment and planning retreatment; however, further studies are required.
Keywords: 223Ra-Dichloride; Alkaline phosphatase; Bone metastases; Metastatic castration-resistant prostate cancer; Radium; Referral patterns.
Conflict of interest statement
Conflicts of interest
Dr. Dizdarevic provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Dr. Robinson provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Ms. Jessop provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Mr. Begley has, on one occasion, received conference/travel sponsorship from Bayer.
Dr. Main has no conflicts of interest to declare.
Ethical approval
Not applicable – the study falls under clinical audit/quality improvement project or service evaluation and is therefore not considered research requiring NHS ethical approval, as determined by the Medical Research Council Health Authority tool (
Informed consent
All patients gave written informed consent for their diagnostic and therapeutic management and follow up.
Figures
References
-
- Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32:3436–3448. doi: 10.1200/JCO.2013.54.8404. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

