Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results
- PMID: 29998598
- PMCID: PMC6175436
- DOI: 10.1111/epi.14477
Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results
Abstract
Objective: Since 2014, cannabidiol (CBD) has been administered to patients with treatment-resistant epilepsies (TREs) in an ongoing expanded-access program (EAP). We report interim results on the safety and efficacy of CBD in EAP patients treated through December 2016.
Methods: Twenty-five US-based EAP sites enrolling patients with TRE taking stable doses of antiepileptic drugs (AEDs) at baseline were included. During the 4-week baseline period, parents/caregivers kept diaries of all countable seizure types. Patients received oral CBD starting at 2-10 mg/kg/d, titrated to a maximum dose of 25-50 mg/kg/d. Patient visits were every 2-4 weeks through 16 weeks and every 2-12 weeks thereafter. Efficacy endpoints included the percentage change from baseline in median monthly convulsive and total seizure frequency, and percentage of patients with ≥50%, ≥75%, and 100% reductions in seizures vs baseline. Data were analyzed descriptively for the efficacy analysis set and using the last-observation-carried-forward method to account for missing data. Adverse events (AEs) were documented at each visit.
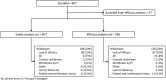
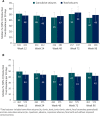
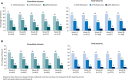
Results: Of 607 patients in the safety dataset, 146 (24%) withdrew; the most common reasons were lack of efficacy (89 [15%]) and AEs (32 [5%]). Mean age was 13 years (range, 0.4-62). Median number of concomitant AEDs was 3 (range, 0-10). Median CBD dose was 25 mg/kg/d; median treatment duration was 48 weeks. Add-on CBD reduced median monthly convulsive seizures by 51% and total seizures by 48% at 12 weeks; reductions were similar through 96 weeks. Proportion of patients with ≥50%, ≥75%, and 100% reductions in convulsive seizures were 52%, 31%, and 11%, respectively, at 12 weeks, with similar rates through 96 weeks. CBD was generally well tolerated; most common AEs were diarrhea (29%) and somnolence (22%).
Significance: Results from this ongoing EAP support previous observational and clinical trial data showing that add-on CBD may be an efficacious long-term treatment option for TRE.
Keywords: cannabidiol; efficacy; expanded access program; seizures; tolerability; treatment-resistant epilepsy.
© 2018 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures
References
-
- Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. - PubMed
-
- Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol. 2016;15:270–8. - PubMed
-
- Patel A, Devinsky O, Cross JH, et al. Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox‐Gastaut syndrome (LGS): results of a dose‐ranging, multi‐center, randomized, double‐blind, placebo‐controlled trial (GWPCARE3). Neurology. 2017;89:e100.
-
- Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. - PubMed
-
- Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

