Microfluidic fabrication of microparticles for biomedical applications
- PMID: 29999050
- PMCID: PMC6140344
- DOI: 10.1039/c7cs00263g
Microfluidic fabrication of microparticles for biomedical applications
Abstract
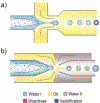
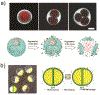
Droplet microfluidics offers exquisite control over the flows of multiple fluids in microscale, enabling fabrication of advanced microparticles with precisely tunable structures and compositions in a high throughput manner. The combination of these remarkable features with proper materials and fabrication methods has enabled high efficiency, direct encapsulation of actives in microparticles whose features and functionalities can be well controlled. These microparticles have great potential in a wide range of bio-related applications including drug delivery, cell-laden matrices, biosensors and even as artificial cells. In this review, we briefly summarize the materials, fabrication methods, and microparticle structures produced with droplet microfluidics. We also provide a comprehensive overview of their recent uses in biomedical applications. Finally, we discuss the existing challenges and perspectives to promote the future development of these engineered microparticles.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures








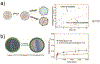










References
-
- Hernandez RM, Orive G, Murua A and Pedraz JL, Adv. Drug Deliv. Rev, 2010, 62, 711–730. - PubMed
-
- Choi A, Seo KD, Kim DW, Kim BC and Kim DS, Lab Chip, 2017, 17, 591–613. - PubMed
-
- Shang L, Cheng Y and Zhao Y, Chem. Rev, 2017, 117, 7964–8040. - PubMed
-
- Tran VT, Benoit JP and Venier-Julienne MC, Int. J. Pharm, 2011, 407, 1–11. - PubMed
-
- Thiele J, Macromol. Chem. Phys, 2017, 218, 1600429.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

