Mass Spectrometry Reveals a Multifaceted Role of Glycosaminoglycan Chains in Factor Xa Inactivation by Antithrombin
- PMID: 29999301
- PMCID: PMC6445383
- DOI: 10.1021/acs.biochem.8b00199
Mass Spectrometry Reveals a Multifaceted Role of Glycosaminoglycan Chains in Factor Xa Inactivation by Antithrombin
Abstract
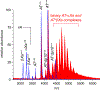
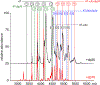
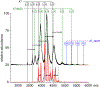
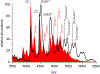
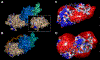
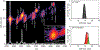
Factor Xa (fXa) inhibition by antithrombin (AT) enabled by heparin or heparan sulfate is critical for controlling blood coagulation. AT activation by heparin has been investigated extensively, while interaction of heparin with trapped AT/fXa intermediates has received relatively little attention. We use native electrospray ionization mass spectrometry to study the role of heparin chains of varying length [hexa-, octa-, deca-, and eicosasaccharides (dp6, dp8, dp10, and dp20, respectively)] in AT/fXa complex assembly. Despite being critical promoters of AT/Xa binding, shorter heparin chains are excluded from the final products (trapped intermediates). However, replacement of short heparin segments with dp20 gives rise to a prominent ionic signal of ternary complexes. These species are also observed when the trapped intermediate is initially prepared in the presence of a short oligoheparin (dp6), followed by addition of a longer heparin chain (dp20), indicating that binding of heparin to AT/fXa complexes takes place after the inhibition event. The importance of the heparin chain length for its ability to associate with the trapped intermediate suggests that the binding likely occurs in a bidentate fashion (where two distinct segments of oligoheparin make contacts with the protein components, while the part of the chain separating these two segments is extended into solution to minimize electrostatic repulsion). This model is corroborated by both molecular dynamics simulations with an explicit solvent and ion mobility measurements in the gas phase. The observed post-inhibition binding of heparin to the trapped AT/fXa intermediates hints at the likely role played by heparan sulfate in their catabolism.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing financial interest.
Figures


References
-
- Howell WH, and Holt E (1918) Two new factors in blood coagulation ‐ heparin and pro‐antithrombin, Am. J. Physiol 47, 328–341.
-
- Messmore HL (1986) Clinical efficacy of heparin fractions: issues and answers, Crit. Rev. Clin. Lab. Sci 23, 77–94. - PubMed
-
- Petitou M, and van Boeckel CAA (2004) A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next?, Angew. Chem. Int. Ed. Engl 43, 3118–3133. - PubMed
-
- Di Nisio M, Middeldorp S, and Büller HR (2005) Direct Thrombin Inhibitors, N. Engl. J. Med 353, 1028–1040. - PubMed
-
- Versteeg HH, Heemskerk JWM, Levi M, and Reitsma PH (2013) New Fundamentals in Hemostasis, Physiol. Rev 93, 327–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

