Does experience provide a permissive or instructive influence on the development of direction selectivity in visual cortex?
- PMID: 30001203
- PMCID: PMC6044012
- DOI: 10.1186/s13064-018-0113-x
Does experience provide a permissive or instructive influence on the development of direction selectivity in visual cortex?
Abstract
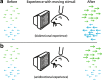
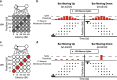
In principle, the development of sensory receptive fields in cortex could arise from experience-independent mechanisms that have been acquired through evolution, or through an online analysis of the sensory experience of the individual animal. Here we review recent experiments that suggest that the development of direction selectivity in carnivore visual cortex requires experience, but also suggest that the experience of an individual animal cannot greatly influence the parameters of the direction tuning that emerges, including direction angle preference and speed tuning. The direction angle preference that a neuron will acquire can be predicted from small initial biases that are present in the naïve cortex prior to the onset of visual experience. Further, experience with stimuli that move at slow or fast speeds does not alter the speed tuning properties of direction-selective neurons, suggesting that speed tuning preferences are built in. Finally, unpatterned optogenetic activation of the cortex over a period of a few hours is sufficient to produce the rapid emergence of direction selectivity in the naïve ferret cortex, suggesting that information about the direction angle preference that cells will acquire must already be present in the cortical circuit prior to experience. These results are consistent with the idea that experience has a permissive influence on the development of direction selectivity.
Keywords: Area 17; Development; Motion; Striate cortex; Thalamocortical.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Katz DB, Sadacca BF. Taste. In: Gottfried JA, editor. SourceNeurobiology of sensation and reward. Baco Raton, FL: CRC Press; 2011.
-
- Wolf M. Proust and the squid: the story and science of the reading brain. NY: HarperCollins; 2007.
-
- Allman JM. Evolving brains. New York: Scientific American Library; 1999.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

