Alternative DNA secondary structure formation affects RNA polymerase II promoter-proximal pausing in human
- PMID: 30001206
- PMCID: PMC6042338
- DOI: 10.1186/s13059-018-1463-8
Alternative DNA secondary structure formation affects RNA polymerase II promoter-proximal pausing in human
Abstract
Background: Alternative DNA secondary structures can arise from single-stranded DNA when duplex DNA is unwound during DNA processes such as transcription, resulting in the regulation or perturbation of these processes. We identify sites of high propensity to form stable DNA secondary structure across the human genome using Mfold and ViennaRNA programs with parameters for analyzing DNA.
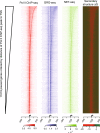
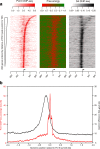
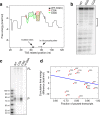
Results: The promoter-proximal regions of genes with paused transcription are significantly and energetically more favorable to form DNA secondary structure than non-paused genes or genes without RNA polymerase II (Pol II) binding. Using Pol II ChIP-seq, GRO-seq, NET-seq, and mNET-seq data, we arrive at a robust set of criteria for Pol II pausing, independent of annotation, and find that a highly stable secondary structure is likely to form about 10-50 nucleotides upstream of a Pol II pausing site. Structure probing data confirm the existence of DNA secondary structures enriched at the promoter-proximal regions of paused genes in human cells. Using an in vitro transcription assay, we demonstrate that Pol II pausing at HSPA1B, a human heat shock gene, is affected by manipulating DNA secondary structure upstream of the pausing site.
Conclusions: Our results indicate alternative DNA secondary structure formation as a mechanism for how GC-rich sequences regulate RNA Pol II promoter-proximal pausing genome-wide.
Keywords: DNA secondary structure; RNA polymerase II promoter-proximal pausing.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
LCTP is a full-time employee of Relay Therapeutics. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

