Preservation of kidney function in kidney transplant recipients by alkali therapy (Preserve-Transplant Study): rationale and study protocol
- PMID: 30001705
- PMCID: PMC6043955
- DOI: 10.1186/s12882-018-0956-8
Preservation of kidney function in kidney transplant recipients by alkali therapy (Preserve-Transplant Study): rationale and study protocol
Abstract
Background: Graft survival after kidney transplantation has significantly improved within the last decades but there is a substantial number of patients with declining transplant function and graft loss. Over the past years several studies have shown that metabolic acidosis plays an important role in the progression of Chronic Kidney Disease (CKD) and that alkalinizing therapies significantly delayed progression of CKD. Importantly, metabolic acidosis is highly prevalent in renal transplant patients and a recent retrospective study has shown that metabolic acidosis is associated with increased risk of graft loss and patient death in kidney transplant recipients. However, no prospective trial has been initiated yet to test the role of alkali treatment on renal allograft function.
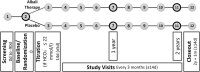
Methods: The Preserve-Transplant Study is an investigator-initiated, prospective, patient-blinded, multi-center, randomized, controlled phase-IV trial with two parallel-groups comparing sodium bicarbonate to placebo. The primary objective is to test if alkali treatment will preserve kidney graft function and diminish the progression of CKD in renal transplant patients by assesing the change in eGFR over 2 years from baseline. Additionally we want to investigate the underlying pathomechanisms of nephrotoxicity of metabolic acidosis.
Discussion: This study has the potential to provide evidence that alkali treatment may slow or reduce the progression towards graft failure and significantly decrease the rate of end stage renal disease (ESRD), thus prolonging long-term graft survival. The implementation of alkali therapy into the drug regimen of kidney transplant recipients would have a favorable risk-benefit ratio since alkali supplements are routinely used in CKD patients and represent a well-tolerated, safe and cost-effective treatment.
Trial registration: ClinicalTrials.gov NCT03102996 . Trial registration was completed on April 6, 2017.
Keywords: Graft function; Graft outcome; Metabolic acidosis; Sodium bicarbonate; eGFR.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the lead cantonal ethics committee in Zurich (Switzerland) and the cantonal ethics committee in Berne (Switzerland) and Geneva (Switzerland), Registration number: 2016–02012.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- U.S. Department of Health & Human Services. Am J Transplant. 2014;14(Suppl 1):11-44. 10.1111/ajt.12579, http://srtr.transplant.hrsa.gov/annualreports/2012/pdf/01kidney_13.pdf.
-
- Collaborative Transplant Study [www.ctstransplant.org].
-
- Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl. 2005;(95):S21–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous