IL-33 receptor (ST2) deficiency downregulates myeloid precursors, inflammatory NK and dendritic cells in early phase of sepsis
- PMID: 30001716
- PMCID: PMC6044035
- DOI: 10.1186/s12929-018-0455-z
IL-33 receptor (ST2) deficiency downregulates myeloid precursors, inflammatory NK and dendritic cells in early phase of sepsis
Abstract
Background: Sepsis is a life-threatening disease mediated by profound disturbances in systemic inflammatory response to infection. IL-33 is multifunctional regulator of numerous aspects of innate and adaptive immune response. The aim of this article was to further evaluate the role of IL-33 receptor (ST2) in different pathways of innate immunity during early polymicrobial sepsis.
Methods: Polymicrobial sepsis was induced using cecal ligation and puncture (CLP) model in ST2 deficient (ST2-/-) and wild type BALB/c mice. Peritoneal and spleen cells were isolated for further phenotyping. Apoptosis was determined by immunohistochemistry and flow cytometry.
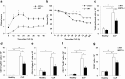
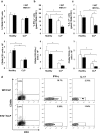
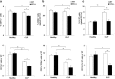
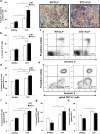
Results: Deletion of ST2 leads to increased susceptibility to early manifestations of sepsis as evaluated by clinical signs and survival. These are accompanied by decrease in the total number of neutrophils, eosinophils and mast cells in peritoneal cavity 12 h after CLP. In early sepsis there was also low number of precursors of myeloid cells in particular CD11b+Ly6G+Ly6Clow cells in spleen of ST2-/- mice. Although the number of NK cells in the spleen was similar, there were significant differences in the presence of inflammatory IFN-γ and IL-17 producing NK cells. Further, ST2 deletion affects the phenotype and maturation of dendritic cell in sepsis. The total number of dendritic cells in the spleen was lower as well as IL-12 expressing dendritic cells. Finally, there was higher frequency of active caspase-3 positive and early apoptotic cells, in particular CD11c positive cells, in spleen of septic ST2-/- mice.
Conclusion: Taken together, our data provide the evidence that ST2 deficiency in early phase of sepsis downregulates myeloid precursors, inflammatory NK and dendritic cells.
Keywords: Dendritic cells; IL-33; Myeloid precursors; NK cells; ST2; Sepsis.
Conflict of interest statement
Ethics approval and consent to participate
All animal procedures were approved by the Ethical Committee of the Faculty of Medical Sciences, University of Kragujevac, Serbia (No 01–10873/3).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

