The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension
- PMID: 30002102
- PMCID: PMC6340636
- DOI: 10.1183/13993003.00589-2018
The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension
Abstract
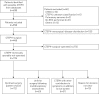
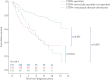
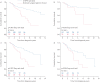
Pulmonary endarterectomy (PEA) is the gold standard treatment for operable chronic thromboembolic pulmonary hypertension (CTEPH). However, a proportion of patients with operable disease decline surgery. There are currently no published data on this patient group. The aim of this study was to identify outcomes and prognostic factors in a large cohort of consecutive patients with CTEPH.Data were collected for consecutive, treatment-naive CTEPH patients at the Pulmonary Vascular Disease Unit of the Royal Hallamshire Hospital (Sheffield, UK) between 2001 and 2014.Of 550 CTEPH patients (mean±sd age 63±15 years, follow-up 4±3 years), 49% underwent surgery, 32% had technically operable disease and did not undergo surgery (including patient choice n=72 and unfit for surgery n=63), and 19% had inoperable disease due to disease distribution. The 5-year survival was superior in patients undergoing PEA (83%) versus technically operable disease who did not undergo surgery (53%) and inoperable due to disease distribution (59%) (p<0.001). Survival was superior in patients following PEA compared with those offered but declining surgery (55%) (p<0.001). In patients offered PEA, independent prognostic factors included mixed venous oxygen saturation, gas transfer and patient decision to proceed to surgery.Outcomes in CTEPH following PEA are excellent and superior to patients declining surgery, and strongly favour consideration of a surgical intervention in eligible patients.
Trial registration: ClinicalTrials.gov NCT02565030.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: A.A.R. Thompson reports nonfinancial support (travel grants) from Actelion Pharmaceuticals Ltd, outside the submitted work. Conflict of interest: C.A. Elliot reports personal fees for lecturing and advisory board work from Actelion Pharmaceuticals, GlaxoSmithKline and Bayer, grants for research from Pfizer, Actelion Pharmaceuticals and Bayer, and grants for travel and attendance at conferences from Bayer and Actelion Pharmaceuticals, outside the submitted work. Conflict of interest: J. Hurdman was part funded as a clinical research fellow by an unrestricted educational grant from Actelion, and has received funding to attend conferences from Actelion, GSK and Pfizer. Conflict of interest: A. Charalampopoulos has received honoraria for lecturing and taking part in advisory boards, as well as support to attend scientific events/congresses from Actelion, GSK, Servier and MSD. Conflict of interest: N. Hamilton has and continues to receive honoraria for participation in advisory boards and educational meetings, and has and continues to receive funding to attend educational meetings from a number of pharmaceutical companies including Actelion, Bayer, GSK and MSD (these companies manufacture drug therapies for a variety of indications, but include areas of clinical interest in pulmonary hypertension). Conflict of interest: J. Pepke-Zaba, or her institution, has received research, educational grants and speaker's honoraria from Actelion, Bayer Pharma AG, Merck and GSK. Conflict of interest: D.P. Jenkins reports personal fees for lecturing from Bayer, and personal fees for lecturing and consultancy work for the MERIT study from Actelion, outside the submitted work. Conflict of interest: A. Lawrie reports research grants from the British Heart Foundation, Medical Research Council UK, Actelion Pharmaceuticals and GSK, and conference travel and scientific meeting support from Actelion Pharmaceuticals, outside the submitted work. Conflict of interest: R. Condliffe has received honoraria for lecturing and advisory boards from Actelion, Bayer and GSK, outside the submitted work. Conflict of interest: D.G. Kiely has received funding to attend educational meetings, fees for giving educational lectures and participating in advisory boards, and institutional funding for research from Actelion, Bayer and GSK, and has received funding to attend educational meetings, and fees for giving educational lectures and participating in advisory boards from MSD, outside the submitted work.
Figures
Comment in
-
Pulmonary endarterectomy and the cost of patient refusal.Eur Respir J. 2018 Sep 16;52(3):1801581. doi: 10.1183/13993003.01581-2018. Print 2018 Sep. Eur Respir J. 2018. PMID: 30220650 No abstract available.
-
Decision-making in pulmonary endarterectomy surgery.Eur Respir J. 2019 Jan 10;53(1):1801973. doi: 10.1183/13993003.01973-2018. Print 2019 Jan. Eur Respir J. 2019. PMID: 30630852 No abstract available.
-
Decision-making in pulmonary endarterectomy surgery.Eur Respir J. 2019 Jan 10;53(1):1802138. doi: 10.1183/13993003.02138-2018. Print 2019 Jan. Eur Respir J. 2019. PMID: 30630853 No abstract available.
References
-
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: D92–D99. - PubMed
-
- Lang IM, Simonneau G, Pepke-Zaba JW, et al. Factors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension a case-control study. Thromb Haemost 2013; 110: 83–91. - PubMed
-
- Pepke-Zaba J, Jansa P, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41: 985–990. - PubMed
-
- Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006; 113: 2011–2020. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
