miR-30a Remodels Subcutaneous Adipose Tissue Inflammation to Improve Insulin Sensitivity in Obesity
- PMID: 30002134
- PMCID: PMC6245225
- DOI: 10.2337/db17-1378
miR-30a Remodels Subcutaneous Adipose Tissue Inflammation to Improve Insulin Sensitivity in Obesity
Abstract
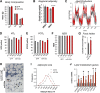
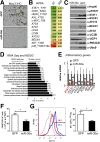
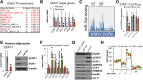
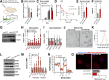
Chronic inflammation accompanies obesity and limits subcutaneous white adipose tissue (WAT) expandability, accelerating the development of insulin resistance and type 2 diabetes mellitus. MicroRNAs (miRNAs) influence expression of many metabolic genes in fat cells, but physiological roles in WAT remain poorly characterized. Here, we report that expression of the miRNA miR-30a in subcutaneous WAT corresponds with insulin sensitivity in obese mice and humans. To examine the hypothesis that restoration of miR-30a expression in WAT improves insulin sensitivity, we injected adenovirus (Adv) expressing miR-30a into the subcutaneous fat pad of diabetic mice. Exogenous miR-30a expression in the subcutaneous WAT depot of obese mice coupled improved insulin sensitivity and increased energy expenditure with decreased ectopic fat deposition in the liver and reduced WAT inflammation. High-throughput proteomic profiling and RNA-Seq suggested that miR-30a targets the transcription factor STAT1 to limit the actions of the proinflammatory cytokine interferon-γ (IFN-γ) that would otherwise restrict WAT expansion and decrease insulin sensitivity. We further demonstrated that miR-30a opposes the actions of IFN-γ, suggesting an important role for miR-30a in defending adipocytes against proinflammatory cytokines that reduce peripheral insulin sensitivity. Together, our data identify a critical molecular signaling axis, elements of which are involved in uncoupling obesity from metabolic dysfunction.
© 2018 by the American Diabetes Association.
Figures


References
-
- Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012. Int J Obes 2016;40:883–886 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

