Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation
- PMID: 30002224
- PMCID: PMC6086708
- DOI: 10.2215/CJN.13811217
Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation
Abstract
Background and objectives: All randomized trials of direct oral anticoagulants in atrial fibrillation excluded patients with severe kidney disease. The safety and effectiveness of direct oral anticoagulants across the range of eGFR in real-world settings is unknown. Our objective is to quantify the risk of bleeding and benefit of ischemic stroke prevention for direct oral anticoagulants compared with warfarin in patients with atrial fibrillation with and without CKD.
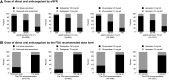
Design, setting, participants, & measurements: We created a propensity score-matched cohort of 3206 patients with atrial fibrillation and direct oral anticoagulant use and 3206 patients with atrial fibrillation using warfarin from October of 2010 to February of 2017 in an electronic health record (Geisinger Health System). The risks of bleeding and ischemic stroke were compared between direct oral anticoagulant and warfarin users using Cox proportional hazards regression, stratified by eGFR (≥60 and <60 ml/min per 1.73 m2).
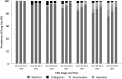
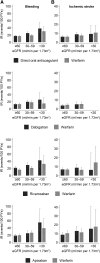
Results: The mean (SD) age of the 6412 participants was 72 (12) years, 47% were women, and average eGFR was 69 (21) ml/min per 1.73 m2. There were 1181 bleeding events and 466 ischemic strokes over 7391 person-years of follow-up. Compared with warfarin use, the hazard ratios (HRs) (95% confidence interval [95% CI]) of bleeding associated with direct oral anticoagulant use were 1.01 (0.88 to 1.17) and 1.23 (1.02 to 1.48) for those with eGFR≥60 and eGFR<60 ml/min per 1.73 m2, respectively (P-interaction=0.10). There was no difference between direct oral anticoagulant and warfarin users in the risk of ischemic stroke: HRs (95% CI) of 0.94 (0.74 to 1.18) and 1.02 (0.76 to 1.37) for those with eGFR≥60 and eGFR<60 ml/min per 1.73 m2, respectively (P-interaction=0.70). Similar findings were observed with individual drugs.
Conclusions: In a large health care system, patients with eGFR<60 ml/min per 1.73 m2 who took direct oral anticoagulants for atrial fibrillation had slightly higher risk of bleeding compared with those on warfarin, but similar benefits from prevention of ischemic stroke.
Keywords: Anticoagulants; Atrial Fibrillation; Brain Ischemia; Confidence Intervals; Direct Oral Anticoagulants; Electronic Health Records; Female; Follow-up Studies; Hemorrhage; Humans; Propensity Score; Renal Insufficiency, Chronic; Risk Assessment; Stroke; Warfarin; chronic kidney disease; glomerular filtration rate; kidney.
Copyright © 2018 by the American Society of Nephrology.
Figures


References
-
- Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG) : 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33: 2719–2747, 2012 - PubMed
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr ., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members : 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130: 2071–2104, 2014 - PubMed
-
- Hart RG, Pearce LA, Aguilar MI: Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146: 857–867, 2007 - PubMed
-
- Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE: Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 349: 1019–1026, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

