Every step of the way: integrins in cancer progression and metastasis
- PMID: 30002479
- PMCID: PMC6629548
- DOI: 10.1038/s41568-018-0038-z
Every step of the way: integrins in cancer progression and metastasis
Erratum in
-
Author Correction: Every step of the way: integrins in cancer progression and metastasis.Nat Rev Cancer. 2019 Mar;19(3):179. doi: 10.1038/s41568-019-0112-1. Nat Rev Cancer. 2019. PMID: 30705430
Abstract
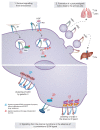
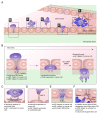
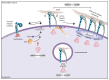
Cell adhesion to the extracellular matrix is fundamental to tissue integrity and human health. Integrins are the main cellular adhesion receptors that through multifaceted roles as signalling molecules, mechanotransducers and key components of the cell migration machinery are implicated in nearly every step of cancer progression from primary tumour development to metastasis. Altered integrin expression is frequently detected in tumours, where integrins have roles in supporting oncogenic growth factor receptor (GFR) signalling and GFR-dependent cancer cell migration and invasion. In addition, integrins determine colonization of metastatic sites and facilitate anchorage-independent survival of circulating tumour cells. Investigations describing integrin engagement with a growing number of versatile cell surface molecules, including channels, receptors and secreted proteins, continue to lead to the identification of novel tumour-promoting pathways. Integrin-mediated sensing, stiffening and remodelling of the tumour stroma are key steps in cancer progression supporting invasion, acquisition of cancer stem cell characteristics and drug resistance. Given the complexity of integrins and their adaptable and sometimes antagonistic roles in cancer cells and the tumour microenvironment, therapeutic targeting of these receptors has been a challenge. However, novel approaches to target integrins and antagonism of specific integrin subunits in stringently stratified patient cohorts are emerging as potential ways forward.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

