Structural flexibility of halogen bonds showed in a single-crystal-to-single-crystal [2+2] photodimerization
- PMID: 30002849
- PMCID: PMC6038960
- DOI: 10.1107/S2052252518007583
Structural flexibility of halogen bonds showed in a single-crystal-to-single-crystal [2+2] photodimerization
Abstract
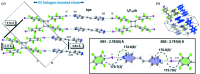
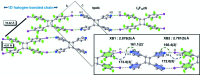
Halogen bonds have emerged as noncovalent forces that govern the assembly of molecules in organic solids with a degree of reliability akin to hydrogen bonds. Although the structure-directing roles of halogen bonds are often compared to hydrogen bonds, general knowledge concerning the fundamental structural behavior of halogen bonds has had limited opportunity to develop. Following an investigation of solid-state reactions involving organic syntheses and the development of photoresponsive materials, this work demonstrates the ability of the components of intermolecular N⋯I halogen bonding - a 'workhorse' interaction for the crystal engineer - to support a single-crystal-to-single-crystal [2+2] photodimerization. A comparison is provided of the geometric changes experienced by the halogen-bonded components in the single-crystal reaction to the current crystal landscape of N⋯I halogen bonds, as derived from the Cambridge Structural Database. Specifically, a linear-to-bent type of deformation of the halogen-bonded components was observed, which is expected to support the development of functional halogen-bonded materials containing molecules that can undergo movements in close-packed crystal environments.
Keywords: co-crystals; crystal engineering; framework-structured solids and amorphous materials; halogen bonds; molecular crystals; organic solid-state reactions; photodimerization; single-crystal-to-single-crystal reaction; solid-state reactivity; supramolecular chemistry.
Figures


References
-
- Barbour, L. J. (2006). Aust. J. Chem. 59, 595–596.
-
- Bhattacharya, S., Stojaković, J., Saha, B. K. & MacGillivray, L. R. (2013). Org. Lett. 15, 744–747. - PubMed
-
- Bruker (2012). APEX2. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruno, I. J., Cole, J. C., Lommerse, J. P. M., Rowland, R. S., Taylor, R. & Verdonk, M. L. (1997). J. Comput. Aided Mol. Des. 11, 525–537. - PubMed
-
- Bushuyev, O. S., Corkery, T. C., Barrett, C. J. & Friščić, T. (2014). Chem. Sci. 5, 3158–3164.
LinkOut - more resources
Full Text Sources
Other Literature Sources

