Synthesis and Characterization of Novel Fluoro-glycosylated Porphyrins that can be Utilized as Theranostic Agents
- PMID: 30003003
- PMCID: PMC6031858
- DOI: 10.1002/open.201800020
Synthesis and Characterization of Novel Fluoro-glycosylated Porphyrins that can be Utilized as Theranostic Agents
Abstract
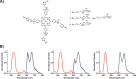
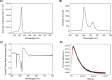
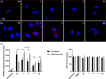
Small molecules with modalities for a variety of imaging techniques as well as therapeutic activity are essential, as such molecules render opportunities to simultaneously conduct diagnosis and targeted therapy, so called theranostics. In this regard, glycoporphyrins have proven useful as theranostic agents towards cancer, as well as noncancerous conditions. Herein, the synthesis and characterization of heterobifunctional glycoconjugated porphyrins with two different sugar moieties, a common monosaccharide at three sites, and a 2-fluoro-2-deoxy glucose (FDG) moiety at the fourth site are presented. The fluoro-glycoconjugated porphyrins exhibit properties for multimodal imaging and photodynamic therapy, as well as specificity towards cancer cells. We foresee that our findings might aid in the chemical design of heterobifunctional glycoconjugated porphyrins that could be utilized as theranostic agents.
Keywords: cancer; glycoporphyrins; imaging; photodynamic therapy; photosensitizers.
Figures



Similar articles
-
Synthesis and Characterization of Novel Fluoro-glycosylated Porphyrins that can be Utilized as Theranostic Agents.ChemistryOpen. 2018 Jun 1;7(7):490. doi: 10.1002/open.201800095. eCollection 2018 Jul. ChemistryOpen. 2018. PMID: 30003001 Free PMC article.
-
Sugar-dependent photodynamic effect of glycoconjugated porphyrins: a study on photocytotoxicity, photophysical properties and binding behavior to bovine serum albumin (BSA).Biochim Biophys Acta. 2007 Aug;1770(8):1204-11. doi: 10.1016/j.bbagen.2007.03.011. Epub 2007 Apr 1. Biochim Biophys Acta. 2007. PMID: 17490818
-
Structure-photodynamic effect relationships of 24 glycoconjugated photosensitizers in HeLa cells.Biol Pharm Bull. 2008 Dec;31(12):2265-72. doi: 10.1248/bpb.31.2265. Biol Pharm Bull. 2008. PMID: 19043211
-
Chemical Synthesis and Medicinal Applications of Glycoporphyrins.Curr Med Chem. 2015;22(19):2238-348. doi: 10.2174/0929867322666150429113104. Curr Med Chem. 2015. PMID: 25921642 Review.
-
Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents.Photodiagnosis Photodyn Ther. 2018 Sep;23:281-294. doi: 10.1016/j.pdpdt.2018.06.023. Epub 2018 Jul 29. Photodiagnosis Photodyn Ther. 2018. PMID: 30009949 Review.
Cited by
-
Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1-Azides, Porphyrins and Corroles.Molecules. 2020 Apr 3;25(7):1662. doi: 10.3390/molecules25071662. Molecules. 2020. PMID: 32260294 Free PMC article. Review.
-
Porphyrin as Diagnostic and Therapeutic Agent.Molecules. 2019 Jul 23;24(14):2669. doi: 10.3390/molecules24142669. Molecules. 2019. PMID: 31340553 Free PMC article. Review.
-
Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment.Int J Mol Sci. 2020 May 9;21(9):3358. doi: 10.3390/ijms21093358. Int J Mol Sci. 2020. PMID: 32397477 Free PMC article. Review.
-
The Self-Aggregation of Porphyrins with Multiple Chiral Centers in Organic/Aqueous Media: The Case of Sugar- and Steroid-Porphyrin Conjugates.Molecules. 2020 Oct 4;25(19):4544. doi: 10.3390/molecules25194544. Molecules. 2020. PMID: 33020381 Free PMC article. Review.
-
Sweetening Pharmaceutical Radiochemistry by 18F-Fluoroglycosylation: Recent Progress and Future Prospects.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1175. doi: 10.3390/ph14111175. Pharmaceuticals (Basel). 2021. PMID: 34832957 Free PMC article. Review.
References
-
- Kojima R., Aubel D., Fussenegger M., Curr. Opin. Chem. Biol. 2015, 28, 29–38. - PubMed
-
- Ethirajan M., Chen Y., Joshi P., Pandey R. K., Chem. Soc. Rev. 2011, 40, 340–362. - PubMed
-
- Chilakamarthi U., Giribabu L., Chem. Rec. 2017, 17, 775–802. - PubMed
-
- Maillard P., Loock B., Grierson D. S., Laville I., Blais J., Doz F., Desjardins L., Carrez D., Guerquin-Kern J. L., Croisy A., Photodiagn. Photodyn. Ther. 2007, 4, 261–268. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

