Hei-Gu-Teng Zhuifenghuoluo Granule Modulates IL-12 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis
- PMID: 30003114
- PMCID: PMC5996447
- DOI: 10.1155/2018/8474867
Hei-Gu-Teng Zhuifenghuoluo Granule Modulates IL-12 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis
Abstract
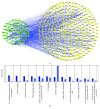
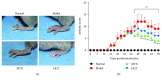
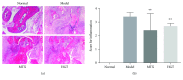
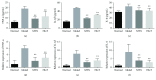
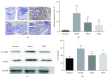
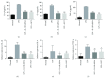
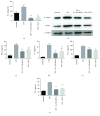
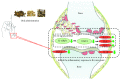
Rheumatoid arthritis (RA) is a type of chronic systemic inflammatory disease; it has a very complicated pathogenesis, and multiple pathological changes are implicated. Traditional Chinese medicine (TCM) like Tripterygium wilfordii Hook. F. or Sinomenium acutum (Thunb.) Rehd et Wils. has been extensively used for centuries in the treatment of arthritic diseases and been reported effective for relieving the severity of RA. Hei-Gu-Teng Zhuifenghuoluo granule (HGT) which contains Periploca forrestii Schltr., Sinomenium acutum (Thunb.) Rehd et Wils., and Lysimachia paridiformis Franch. var. stenophylla Franch. was a representative natural rattan herb formula for the treatment of RA in China, but the mechanism has not been elucidated. This study aimed at exploring the mechanism of HGT on RA using the bioinformatics analysis with in vivo and in vitro experiment validation. The potential action mechanism was first investigated by bioinformatics analysis via Ingenuity Pathway Analysis (IPA) software. After that, we use experimental validation such as collagen-induced arthritis (CIA) mice model in vivo and U937 cell model in vitro. The bioinformatics results suggested that HGT may have anti-inflammatory characteristic on RA and IL-12 signaling pathway could be the potential key trigger. In vivo experiments demonstrated that HGT ameliorated the symptoms in CIA mice and decreased the production of inflammatory cytokines in both mice ankle joints and serum. Furthermore, HGT effectively inhibited the activation of IL-12R and STAT4 on IL-12 signaling pathway. In vitro experiments showed that HGT inhibited the production of IL-12R and STAT4 induced by IL-12 in lipopolysaccharide- (LPS-) stimulated U937 cells. Moreover, IL-12R knockdown was able to interfere with the inhibition effects of HGT on the production of these cytokines. Our results confirmed the anti-inflammatory property of HGT, which was attributed to its inhibition on IL-12 signaling pathway.
Figures
Similar articles
-
Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis.Int J Mol Sci. 2016 Apr 2;17(4):498. doi: 10.3390/ijms17040498. Int J Mol Sci. 2016. PMID: 27049384 Free PMC article.
-
Therapeutic effects of total alkaloids of Tripterygium wilfordii Hook f. on collagen-induced arthritis in rats.J Ethnopharmacol. 2013 Feb 13;145(3):699-705. doi: 10.1016/j.jep.2012.11.018. Epub 2012 Nov 23. J Ethnopharmacol. 2013. PMID: 23183089
-
Tibetan medicine Kuan-Jin-Teng exerts anti-arthritic effects on collagen-induced arthritis rats via inhibition the production of pro-inflammatory cytokines and down-regulation of MAPK signaling pathway.Phytomedicine. 2019 Apr;57:271-281. doi: 10.1016/j.phymed.2018.12.023. Epub 2018 Dec 18. Phytomedicine. 2019. PMID: 30802713
-
[Study progress in Sinomenium acutum (Thunb.) Rehd. et Wils].Zhong Yao Cai. 2002 Mar;25(3):209-11. Zhong Yao Cai. 2002. PMID: 12583167 Review. Chinese.
-
Sinomenium acutum: A Comprehensive Review of its Botany, Phytochemistry, Pharmacology and Clinical Application.Am J Chin Med. 2022;50(5):1219-1253. doi: 10.1142/S0192415X22500501. Epub 2022 Jun 8. Am J Chin Med. 2022. PMID: 35681262 Review.
Cited by
-
Perspectives of herbs and their natural compounds, and herb formulas on treating diverse diseases through regulating complicated JAK/STAT signaling.Front Pharmacol. 2022 Oct 17;13:993862. doi: 10.3389/fphar.2022.993862. eCollection 2022. Front Pharmacol. 2022. PMID: 36324680 Free PMC article. Review.
-
Piacentinu Ennese PDO Cheese as Reservoir of Promising Probiotic Bacteria.Microorganisms. 2019 Aug 12;7(8):254. doi: 10.3390/microorganisms7080254. Microorganisms. 2019. PMID: 31408976 Free PMC article.
-
Saponin from Periploca forrestii Schltr Mitigates Oxazolone-Induced Atopic Dermatitis via Modulating Macrophage Activation.Mediators Inflamm. 2020 Oct 15;2020:4346367. doi: 10.1155/2020/4346367. eCollection 2020. Mediators Inflamm. 2020. PMID: 33122966 Free PMC article.
-
Efficacy and Safety of Acupuncture Therapy for Patients with Acute Ankle Sprain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Evid Based Complement Alternat Med. 2020 Oct 16;2020:9109531. doi: 10.1155/2020/9109531. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33123213 Free PMC article.
-
Traditional Chinese medicine on treating active rheumatoid arthritis: A protocol for systematic review and meta-analysis.Medicine (Baltimore). 2020 Jun 12;99(24):e20642. doi: 10.1097/MD.0000000000020642. Medicine (Baltimore). 2020. PMID: 32541503 Free PMC article.
References
-
- Atkinson S. M., Usher P. A., Kvist P. H., Markholst H., Haase C., Nansen A. Establishment and characterization of a sustained delayed-type hypersensitivity model with arthritic manifestations in C57BL/6J mice. Arthritis Research & Therapy. 2012;14(3):p. R134. doi: 10.1186/ar3867. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

