Personalized Medicine in the Oncology Clinic: Implementation and Outcomes of the Johns Hopkins Molecular Tumor Board
- PMID: 30003184
- PMCID: PMC6039131
- DOI: 10.1200/PO.16.00046
Personalized Medicine in the Oncology Clinic: Implementation and Outcomes of the Johns Hopkins Molecular Tumor Board
Abstract
Purpose: Tumor genomic profiling for personalized oncology therapy is being widely applied in clinical practice even as it is being evaluated more formally in clinical trials. Given the complexities of genomic data and its application to clinical use, molecular tumor boards with diverse expertise can provide guidance to oncologists and patients seeking to implement personalized genetically targeted therapy in practice.
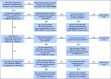
Methods: A multidisciplinary molecular tumor board reviewed tumor molecular profiling reports from consecutive referrals at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins over a 3-year period. The tumor board weighed evidence for actionability of genomic alterations identified by molecular profiling and provided recommendations including US Food and Drug Administration-approved drug therapy, clinical trials of matched targeted therapy, off-label use of such therapy, and additional tumor or germline genetic testing.
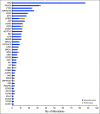
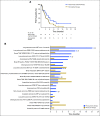
Results: One hundred fifty-five patients were reviewed. Actionable genomic alterations were identified in 132 patients (85%). Off-label therapies were recommended in 37 patients (24%). Eleven patients were treated off-label, and 13 patients were enrolled onto clinical trials of matched targeted therapies. Median progression-free survival of patients treated with matched therapies was 5 months (95% CI, 2.9 months to not reached), and the progression-free survival probability at 6 months was 43%(95% CI, 26% to 71%). Lack of locally available clinical trials was the major limitation on clinical actionability of tumor profiling reports.
Conclusion: The molecular tumor board recommended off-label targeted therapies for a quarter of all patients reviewed. Outcomes were heterogeneous, although 43% of patients receiving genomically matched therapy derived clinical benefit lasting at least 6 months. Until more data become available from precision oncology trials, molecular tumor boards can help guide appropriate use of tumor molecular testing to direct therapy.
Conflict of interest statement
Personalized Medicine in the Oncology Clinic: Implementation and Outcomes of the Johns Hopkins Molecular Tumor Board
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
W. Brian Dalton
No relationship to disclose
Patrick M. Forde
Hyunseok Kang
Roisin M. Connolly
Vered Stearns
Christopher D. Gocke
No relationship to disclose
James R. Eshleman
No relationship to disclose
Jennifer Axilbund
Dana Petry
No relationship to disclose
Cindy Geoghegan
Antonio C. Wolff
David M. Loeb
No relationship to disclose
Christine A. Pratilas
Christian F. Meyer
Eric S. Christenson
No relationship to disclose
Shannon A. Slater
No relationship to disclose
Jennifer Ensminger
No relationship to disclose
Heather A. Parsons
No relationship to disclose
Ben H. Park
Josh Lauring
Figures

References
-
- Conley BA, Doroshow JH: Molecular analysis for therapy choice: NCI MATCH. Semin Oncol 41:297-299, 2014 - PubMed
-
- Kurzrock R, Colevas AD, Olszanski A, et al. : NCCN Oncology Research Program’s Investigator Steering Committee and NCCN Best Practices Committee molecular profiling surveys. J Natl Compr Canc Netw 13:1337-1346, 2015 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

