Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse
- PMID: 30003233
- PMCID: PMC6143013
- DOI: 10.1001/jamaoncol.2018.2091
Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse
Abstract
Importance: The optimal treatment approach to patients with acute myeloid leukemia (AML) who relapse after an allogeneic hematopoietic cell transplant (allo-HCT) remains elusive. No randomized clinical trial comparing survival outcomes of a second allo-HCT (allo-HCT2) vs donor lymphocyte infusion (DLI) has been conducted to date.
Objective: To compare overall survival (OS) after an allo-HCT2 or DLI in relapsed AML after a first allo-HCT.
Design, setting, and participants: A retrospective registry study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation involving 418 adults who received an allo-HCT2 (n = 137) or DLI (n = 281) for postallograft-relapsed AML. Analysis was assessed on the principle of intent-to-first received intervention. The data were collected from November 21, 2015, to May 15, 2017, and analysis was performed June 1, 2017.
Main outcomes and measures: Number of patients with relapsed AML who are alive after 2 years and 5 years from receiving an allo-HCT2 or DLI.
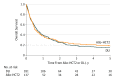
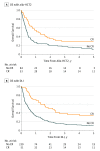
Results: Of the 418 patients, 228 (54.5%) were men; mean age was 46.2 years (interquartile range, 36.5-56.9 years). There was no apparent difference in OS whether an allo-HCT2 or DLI was prescribed (2-year OS with allo-HCT2, 26%; 5-year OS with allo-HCT2, 19%; 2-year OS with DLI, 25%; 5-year OS with DLI, 15%; P = .86). Overall survival was better if either of these procedures was offered when the patient was in complete remission (hazard ratio, 0.55; 95% CI, 0.41-0.74; P < .001). Conversely, OS was low for patients relapsing within less than 6 months after an allo-HCT1, regardless of the treatment prescribed (5-year OS: allo-HCT2, 9%; 95% CI, 1%-17% vs DLI, 4%; 95% CI, 1%-8%; P = .86).
Conclusion and relevance: Heterogeneity of the patient-, disease-, and treatment-related characteristics limit the ability to recommend one approach over another. Findings of this study highlight that best outcomes seem to be achieved in patients relapsing 6 or more months from an allo-HCT1 or those in complete remission at the time of either allo-HCT2 or DLI.
Conflict of interest statement
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

