What is the quality of drug safety information for patients: An analysis of REMS educational materials
- PMID: 30003610
- PMCID: PMC6646909
- DOI: 10.1002/pds.4614
What is the quality of drug safety information for patients: An analysis of REMS educational materials
Abstract
Background: Poor-quality patient drug information has been identified as a major cause of preventable medication errors in the United States. The US Food and Drug Administration (FDA) has the authority to require marketing authorization holders of medicinal products to implement risk evaluation and mitigation strategies (REMS) to ensure that the benefits of a drug or biological product outweigh its risks. Aside from medication guides, no research has been conducted to assess the quality of patient-targeted REMS materials, including whether, and to what extent, patients find these materials understandable and actionable.
Purpose: To describe the readability, understandability, and actionability of patient educational materials in currently approved REMS programs, and to highlight opportunities for improving both the quality and effectiveness of these important drug safety tools.
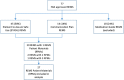
Methods: Seventy-seven REMS programs were identified from the FDA REMS database. We excluded medication guides (MGs) from our analysis because of the fact that there is a mandatory MG template. Based on this, we identified a total of 27 (non-MG) REMS patient materials on the FDA REMS website for analysis purposes. The materials were tested for readability using the Lexile Measure, the Gunning Fog Index, and Flesch Kincaid and then assessed using the Patient Education Materials Assessment Tool for printable materials, for understandability and actionability.
Results: Twenty-three of 77 (30%) REMS programs used educational materials to communicate serious risks to patients, yielding a total of 27 REMS patient materials for analysis. The median readability score for these materials was at a ninth-grade reading level or higher. While most (89%) of these patient education materials met established criteria for being understandable, less than half (49%) were deemed actionable.
Discussion: Currently approved REMS patient materials fell short in terms of recommended reading level, and over half did not meet recommended standards for actionability. Developers of these materials should apply plain language principles when design these materials to improve their readability and to assess both understandability and actionability in order to increase the effectiveness when distributed to patients.
Keywords: drug safety; health literacy; patient labeling; patients; pharmaceutical; pharmacoepidemiology; prescription medicines; risk communication; risk evaluation and mitigation strategy (REMS); risk minimization; therapeutic risk management.
© 2018 John Wiley & Sons, Ltd.
Conflict of interest statement
The views expressed in this publication represent those of the authors and do not necessarily represent the views or practices of the authors' employer or any other third party.
Figures



References
-
- US DHHS . National Action Plan to Improve Health Literacy. DC: Washington; 2011.
-
- PLAIN . Federal Plain Language Guidelines. Plain language action and information Network; 2011.
-
- Aspden P, Wolcott J, Bootman JL, Cronenwett LR. Preventing Medication Errors. Institute of Medicine. Washington, DC: National Academies Press; 2006.
-
- Davis TC, Wolf MS, Bass PF 3rd, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887‐894. - PubMed
-
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97‐107. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

