Glycaemic control and hypoglycaemia benefits with insulin glargine 300 U/mL extend to people with type 2 diabetes and mild-to-moderate renal impairment
- PMID: 30003642
- PMCID: PMC6282564
- DOI: 10.1111/dom.13470
Glycaemic control and hypoglycaemia benefits with insulin glargine 300 U/mL extend to people with type 2 diabetes and mild-to-moderate renal impairment
Abstract
Aim: To investigate the impact of renal function on the safety and efficacy of insulin glargine 300 U/mL (Gla-300) and insulin glargine 100 U/mL (Gla-100).
Materials and methods: A meta-analysis was performed using pooled 6-month data from the EDITION 1, 2 and 3 trials (N = 2496). Eligible participants, aged ≥18 years with a diagnosis of type 2 diabetes (T2DM), were randomized to receive once-daily evening injections of Gla-300 or Gla-100. Pooled results were assessed by two renal function subgroups: estimated glomerular filtration rate (eGFR) <60 and ≥60 mL/min/1.73 m2 .
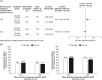
Results: The decrease in glycated haemoglobin (HbA1c) after 6 months and the proportion of individuals with T2DM achieving HbA1c targets were similar in the Gla-300 and Gla-100 groups, for both renal function subgroups. There was a reduced risk of nocturnal (12:00-5:59 am) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia with Gla-300 in both renal function subgroups (eGFR <60 mL/min/1.73 m2 : relative risk [RR] 0.76 [95% confidence interval {CI} 0.62-0.94] and eGFR ≥60 mL/min/1.73 m2 : RR 0.75 [95% CI 0.67-0.85]). For confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycaemia at any time of day (24 hours) the hypoglycaemia risk was lower with Gla-300 vs Gla-100 in both the lower (RR 0.94 [95% CI 0.86-1.03]) and higher (RR 0.90 [95% CI 0.85-0.95]) eGFR subgroups.
Conclusions: Gla-300 provided similar glycaemic control to Gla-100, while indicating a reduced overall risk of confirmed (≤3.9 and <3.0 mmol/L [≤70 and <54 mg/dL]) or severe hypoglycaemia, with no significant difference between renal function subgroups.
Keywords: basal insulin; glycaemic control; hypoglycaemia; insulin analogues; meta-analysis; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
J.E. has participated in advisory panels for MSD, Novo Nordisk and Sanofi, and has participated in speaker's bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi. S.H. has participated in advisory boards, conferences and acted as a consultant for Ascensia, AstraZeneca, Bayer, Becton‐Dickinson, Boehringer Ingelheim, Janssen, Lifescan, Eli Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. P.S. has participated in advisory panels, advisory boards, and acted as a consultant for Abbott, AstraZeneca, Eli Lilly, Genzyme, GlaxoSmithKline, Janssen, Medtronic, Novo Nordisk, Sanofi and Servier, received research support from AstraZeneca, Boehringer Ingelheim, Prometic, Novo Nordisk, Sanofi, Servier and Viacyte, and participated in speaker's bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Lifescan, Novartis, Novo Nordisk, Sanofi and Valeant. M.B. and A.C. are employees of Sanofi. L.M.‐M is an employee of IVIDATA Group, providing consultancy to Sanofi. J.K. has received research support from AstraZeneca and Sanofi, and participated in speaker's bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Janssen. R.R. has acted as a consultant for AstraZeneca, MSD, Novo Nordisk, Sanofi and Servier, and participated in speaker's bureaus for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, MSD, Novartis, Novo Nordisk and Sanofi.
Figures


Similar articles
-
Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes.Diabetes Obes Metab. 2018 Mar;20(3):541-548. doi: 10.1111/dom.13105. Epub 2017 Oct 5. Diabetes Obes Metab. 2018. PMID: 28862801 Free PMC article. Review.
-
Glycaemic control and hypoglycaemia risk with insulin glargine 300 U/mL versus glargine 100 U/mL: A patient-level meta-analysis examining older and younger adults with type 2 diabetes.Diabetes Metab. 2020 Apr;46(2):110-118. doi: 10.1016/j.diabet.2018.10.002. Epub 2018 Oct 23. Diabetes Metab. 2020. PMID: 30366067 Clinical Trial.
-
One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension.Diabetes Obes Metab. 2015 Sep;17(9):835-42. doi: 10.1111/dom.12472. Epub 2015 May 11. Diabetes Obes Metab. 2015. PMID: 25846721 Free PMC article. Clinical Trial.
-
New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2).Diabetes Obes Metab. 2016 Apr;18(4):366-74. doi: 10.1111/dom.12618. Epub 2016 Jan 21. Diabetes Obes Metab. 2016. PMID: 26662838 Free PMC article. Clinical Trial.
-
Effectiveness of insulin glargine U-300 versus insulin glargine U-100 on nocturnal hypoglycemia and glycemic control in type 1 and type 2 diabetes: a systematic review and meta-analysis.Acta Diabetol. 2019 Mar;56(3):355-364. doi: 10.1007/s00592-018-1258-0. Epub 2018 Dec 3. Acta Diabetol. 2019. PMID: 30506484
Cited by
-
The Safety and Efficacy of Second-Generation Basal Insulin Analogues in Adults with Type 2 Diabetes at Risk of Hypoglycemia and Use in Other Special Populations: A Narrative Review.Diabetes Ther. 2020 Nov;11(11):2555-2593. doi: 10.1007/s13300-020-00925-8. Epub 2020 Sep 25. Diabetes Ther. 2020. PMID: 32975710 Free PMC article. Review.
-
How Similar Are Biosimilars? What Do Clinicians Need to Know About Biosimilar and Follow-On Insulins?Clin Diabetes. 2017 Oct;35(4):209-216. doi: 10.2337/cd16-0072. Clin Diabetes. 2017. PMID: 29109610 Free PMC article.
-
Use of Second-Generation Basal Insulin Gla-300 in Special Populations: A Narrative Mini-Review.Curr Diabetes Rev. 2023;19(9):e090123212447. doi: 10.2174/1573399819666230109113205. Curr Diabetes Rev. 2023. PMID: 36624651 Free PMC article. Review.
-
Real-World Persistence, Adherence, Hypoglycemia, and Health Care Resource Utilization in People With Type 2 Diabetes Who Continued With the Second-Generation Basal Insulin Analog Insulin Glargine 300 Units/mL or Switched to a First-Generation Basal Insulin (Insulin Glargine 100 Units/mL or Detemir 100).Clin Diabetes. 2023 Summer;41(3):425-434. doi: 10.2337/cd22-0096. Epub 2023 Mar 28. Clin Diabetes. 2023. PMID: 37456096 Free PMC article.
-
Real-world persistence, adherence, health care resource utilization, and costs in people with type 2 diabetes switching from a first-generation basal insulin to a second-generation (insulin glargine 300 U/mL) vs an alternative first-generation basal insulin.J Manag Care Spec Pharm. 2022 Jun;28(6):592-603. doi: 10.18553/jmcp.2022.21436. Epub 2022 Mar 30. J Manag Care Spec Pharm. 2022. PMID: 35352995 Free PMC article.
References
-
- Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510‐533. - PubMed
-
- New JP, Middleton RJ, Klebe B, et al. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med. 2007;24:364‐369. - PubMed
-
- Nitta K, Okada K, Yanai M, Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013;38:109‐120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

