Genes within Genes in Bacterial Genomes
- PMID: 30003865
- PMCID: PMC11633611
- DOI: 10.1128/microbiolspec.RWR-0020-2018
Genes within Genes in Bacterial Genomes
Abstract
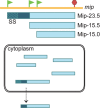
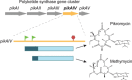
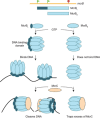
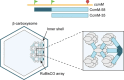
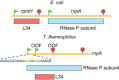
Genetic coding in bacteria largely operates via the "one gene-one protein" paradigm. However, the peculiarities of the mRNA structure, the versatility of the genetic code, and the dynamic nature of translation sometimes allow organisms to deviate from the standard rules of protein encoding. Bacteria can use several unorthodox modes of translation to express more than one protein from a single mRNA cistron. One such alternative path is the use of additional translation initiation sites within the gene. Proteins whose translation is initiated at different start sites within the same reading frame will differ in their N termini but will have identical C-terminal segments. On the other hand, alternative initiation of translation in a register different from the frame dictated by the primary start codon will yield a protein whose sequence is entirely different from the one encoded in the main frame. The use of internal mRNA codons as translation start sites is controlled by the nucleotide sequence and the mRNA folding. The proteins of the alternative proteome generated via the "genes-within-genes" strategy may carry important functions. In this review, we summarize the currently known examples of bacterial genes encoding more than one protein due to the utilization of additional translation start sites and discuss the known or proposed functions of the alternative polypeptides in relation to the main protein product of the gene. We also discuss recent proteome- and genome-wide approaches that will allow the discovery of novel translation initiation sites in a systematic fashion.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

