The Microbiota-Inflammasome Hypothesis of Major Depression
- PMID: 30004130
- PMCID: PMC12180310
- DOI: 10.1002/bies.201800027
The Microbiota-Inflammasome Hypothesis of Major Depression
Abstract
We propose the "microbiota-inflammasome" hypothesis of major depressive disorder (MDD, a mental illness affecting the way a person feels and thinks, characterized by long-lasting feelings of sadness). We hypothesize that pathological shifts in gut microbiota composition (dysbiosis) caused by stress and gut conditions result in the upregulation of pro-inflammatory pathways mediated by the Nod-like receptors family pyrin domain containing 3 (NLRP3) inflammasome (an intracellular platform involved in the activation of inflammatory processes). This upregulation exacerbates depressive symptomatology and further compounds gut dysbiosis. In this review we describe MDD/chronic stress-induced changes in: 1) NLRP3 inflammasome; 2) gut microbiota; and 3) metabolic pathways; and how inflammasome signaling may affect depressive-like behavior and gut microbiota composition. The implication is that novel therapeutic strategies could emerge for MDD and co-morbid conditions. A number of testable predictions surface from this microbiota-gut-inflammasome-brain hypothesis of MDD, using approaches that modulate gut microbiota composition via inflammasome modulation, fecal microbiota transplantation, psychobiotics supplementation, or dietary change.
Keywords: NLRP3; depression; dysbiosis; gut microbiota; inflammasome; probiotics.
© 2018 The Authors. BioEssays Published by WILEY Periodicals, Inc.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures

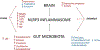
References
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R, Nature 2012, 481, 278. - PubMed
-
- Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullon P, Sanchez-Alcazar JA, Carrion AM, Cordero MD, Mol. Neurobiol. 2016, 53, 4874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

