Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction
- PMID: 30004258
- PMCID: PMC6230908
- DOI: 10.1152/ajpheart.00238.2018
Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction
Abstract
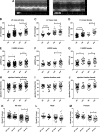
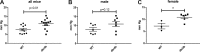
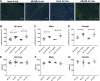
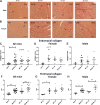
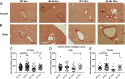
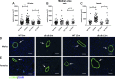
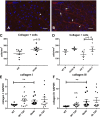
Heart failure with preserved ejection fraction (HFpEF) is caused, or exacerbated by, a wide range of extracardiac conditions. Diabetes, obesity, and metabolic dysfunction are associated with a unique HFpEF phenotype, characterized by inflammation, cardiac fibrosis, and microvascular dysfunction. Development of new therapies for HFpEF is hampered by the absence of reliable animal models. The leptin-resistant db/ db mouse has been extensively studied as a model of diabetes-associated cardiomyopathy; however, data on the functional and morphological alterations in db/ db hearts are conflicting. In the present study, we report a systematic characterization of the cardiac phenotype in db/ db mice, focusing on the time course of functional and histopathological alterations and on the identification of sex-specific cellular events. Although both male and female db/ db mice developed severe obesity, increased adiposity, and hyperglycemia, female mice had more impressive weight gain and exhibited a modest but significant increase in blood pressure. db/ db mice had hypertrophic ventricular remodeling and diastolic dysfunction with preserved ejection fraction; the increase in left ventricular mass was accentuated in female mice. Histological analysis showed that both male and female db/ db mice had cardiomyocyte hypertrophy and interstitial fibrosis, associated with marked thickening of the perimysial collagen, and expansion of the periarteriolar collagen network, in the absence of replacement fibrosis. In vivo and in vitro experiments showed that fibrotic changes in db/ db hearts were associated with increased collagen synthesis by cardiac fibroblasts, in the absence of periostin, α-smooth muscle actin, or fibroblast activation protein overexpression. Male db/ db mice exhibited microvascular rarefaction. In conclusion, the db/ db mouse model recapitulates functional and histological features of human HFpEF associated with metabolic dysfunction. Development of fibrosis in db/ db hearts, in the absence of myofibroblast conversion, suggests that metabolic dysfunction may activate an alternative profibrotic pathway associated with accentuated extracellular matrix protein synthesis. NEW & NOTEWORTHY We provide a systematic analysis of the sex-specific functional and structural myocardial alterations in db/ db mice. Obese diabetic C57BL6J db/ db mice exhibit diastolic dysfunction with preserved ejection fraction, associated with cardiomyocyte hypertrophy, interstitial/perivascular fibrosis, and microvascular rarefaction, thus recapitulating aspects of human obesity-related heart failure with preserved ejection fraction. Myocardial fibrosis in db/ db mice is associated with a matrix-producing fibroblast phenotype, in the absence of myofibroblast conversion, suggesting an alternative mechanism of activation.
Keywords: diabetes; diastolic dysfunction; fibroblast; fibrosis; hypertrophy.
Figures





References
-
- Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res 98: 119–124, 2005. doi: 10.1161/01.RES.0000199348.10580.1d. - DOI - PubMed
-
- Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail 8: 788–798, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001963. - DOI - PMC - PubMed
-
- Buckley LF, Canada JM, Del Buono MG, Carbone S, Trankle CR, Billingsley H, Kadariya D, Arena R, Van Tassell BW, Abbate A. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail 5: 372–378, 2018. doi: 10.1002/ehf2.12235. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

