Role of the heart in blood pressure lowering during chronic baroreflex activation: insight from an in silico analysis
- PMID: 30004810
- PMCID: PMC6297821
- DOI: 10.1152/ajpheart.00302.2018
Role of the heart in blood pressure lowering during chronic baroreflex activation: insight from an in silico analysis
Abstract
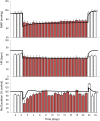
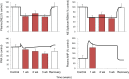
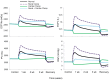
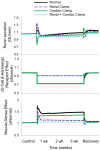
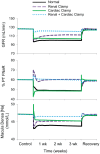
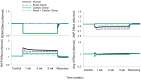
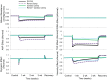
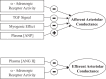
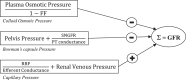
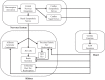
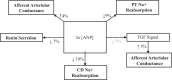
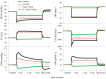
Electrical stimulation of the baroreflex chronically suppresses sympathetic activity and arterial pressure and is currently being evaluated for the treatment of resistant hypertension. The antihypertensive effects of baroreflex activation are often attributed to renal sympathoinhibition. However, baroreflex activation also decreases heart rate, and robust blood pressure lowering occurs even after renal denervation. Because controlling renal sympathetic nerve activity (RSNA) and cardiac autonomic activity cannot be achieved experimentally, we used an established mathematical model of human physiology (HumMod) to provide mechanistic insights into their relative and combined contributions to the cardiovascular responses during baroreflex activation. Three-week responses to baroreflex activation closely mimicked experimental observations in dogs including decreases in blood pressure, heart rate, and plasma norepinephrine and increases in plasma atrial natriuretic peptide (ANP), providing validation of the model. Simulations showed that baroreflex-induced alterations in cardiac sympathetic and parasympathetic activity lead to a sustained depression of cardiac function and increased secretion of ANP. Increased ANP and suppression of RSNA both enhanced renal excretory function and accounted for most of the chronic blood pressure lowering during baroreflex activation. However, when suppression of RSNA was blocked, the blood pressure response to baroreflex activation was not appreciably impaired due to inordinate fluid accumulation and further increases in atrial pressure and ANP secretion. These simulations provide a mechanistic understanding of experimental and clinical observations showing that baroreflex activation effectively lowers blood pressure in subjects with previous renal denervation. NEW & NOTEWORTHY Both experimental and clinical studies have shown that the presence of renal nerves is not an obligate requirement for sustained reductions in blood pressure during chronic electrical stimulation of the carotid baroreflex. Simulations using HumMod, a mathematical model of integrative human physiology, indicated that both increased secretion of atrial natriuretic peptide and suppressed renal sympathetic nerve activity play key roles in mediating long-term reductions in blood pressure during chronic baroreflex activation.
Keywords: atrial natriuretic peptide; baroreflex; blood pressure; modeling; sympathetic nervous system.
Figures



Similar articles
-
Preeminent role of the cardiorenal axis in the antihypertensive response to an arteriovenous fistula: an in silico analysis.Am J Physiol Heart Circ Physiol. 2019 Nov 1;317(5):H1002-H1012. doi: 10.1152/ajpheart.00354.2019. Epub 2019 Aug 30. Am J Physiol Heart Circ Physiol. 2019. PMID: 31469293 Free PMC article.
-
Lowering of blood pressure during chronic suppression of central sympathetic outflow: insight from computer simulations.Clin Exp Pharmacol Physiol. 2010 Feb;37(2):e24-33. doi: 10.1111/j.1440-1681.2009.05291.x. Epub 2009 Sep 21. Clin Exp Pharmacol Physiol. 2010. PMID: 19769610 Free PMC article.
-
Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure.Hypertension. 2007 Feb;49(2):373-9. doi: 10.1161/01.HYP.0000253507.56499.bb. Epub 2006 Dec 11. Hypertension. 2007. PMID: 17159083
-
Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation.J Appl Physiol (1985). 2012 Nov;113(10):1652-8. doi: 10.1152/japplphysiol.00552.2012. Epub 2012 Jul 12. J Appl Physiol (1985). 2012. PMID: 22797307 Free PMC article. Review.
-
The interplay between sympathetic overactivity, hypertension and heart rate variability (review, invited).Acta Physiol Hung. 2014 Jun;101(2):129-42. doi: 10.1556/APhysiol.101.2014.2.1. Acta Physiol Hung. 2014. PMID: 24901074 Review.
Cited by
-
Preeminent role of the cardiorenal axis in the antihypertensive response to an arteriovenous fistula: an in silico analysis.Am J Physiol Heart Circ Physiol. 2019 Nov 1;317(5):H1002-H1012. doi: 10.1152/ajpheart.00354.2019. Epub 2019 Aug 30. Am J Physiol Heart Circ Physiol. 2019. PMID: 31469293 Free PMC article.
-
In silico trial of baroreflex activation therapy for the treatment of obesity-induced hypertension.PLoS One. 2021 Nov 18;16(11):e0259917. doi: 10.1371/journal.pone.0259917. eCollection 2021. PLoS One. 2021. PMID: 34793497 Free PMC article.
-
Device-Based Neuromodulation for Resistant Hypertension Therapy.Circ Res. 2019 Mar 29;124(7):1071-1093. doi: 10.1161/CIRCRESAHA.118.313221. Circ Res. 2019. PMID: 30920919 Free PMC article. Review.
-
Simulation of integrative physiology for medical education.Morphologie. 2019 Dec;103(343):187-193. doi: 10.1016/j.morpho.2019.09.004. Epub 2019 Sep 25. Morphologie. 2019. PMID: 31563456 Free PMC article.
-
Modeling the physiological roles of the heart and kidney in heart failure with preserved ejection fraction during baroreflex activation therapy.Am J Physiol Heart Circ Physiol. 2022 Sep 1;323(3):H597-H607. doi: 10.1152/ajpheart.00329.2022. Epub 2022 Aug 19. Am J Physiol Heart Circ Physiol. 2022. PMID: 35984764 Free PMC article.
References
-
- Baláti B, Phung H, Pousset F, Isnard R, Boisvieux A, Carayon A, Komajda M, Lechat P. Relationships between the antihypertensive effects of bisoprolol and levels of plasma atrial natriuretic peptide in hypertensive patients. Fundam Clin Pharmacol 16: 361–368, 2002. doi:10.1046/j.1472-8206.2002.00072.x. - DOI - PubMed
-
- de Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H, Kroon AA; DEBuT-HT and Rheos Trial Investigators . Sustained reduction of blood pressure with baroreceptor activation therapy: results of the 6-year open follow-up. Hypertension 69: 836–843, 2017. doi:10.1161/HYPERTENSIONAHA.117.09086. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

